Solid-State Synthesis of Direct Z-Scheme Cu2O/WO3 Nanocomposites with Enhanced Visible-Light Photocatalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of As-Prepared Photocatalysts
2.1.1. XRD Analysis
2.1.2. TG/DSC Analysis
2.1.3. Raman Spectroscopy
2.1.4. SEM Analysis
2.1.5. TEM Analysis
2.1.6. BET Surface Area Analysis
2.1.7. UV–VIS Analysis
2.1.8. Electronic Band Structure Analysis
2.2. Evaluation of Photocatalytic Activity under UV and VIS Light
2.3. Photocatalytic Activity Mechanism
2.3.1. Role of Active Species
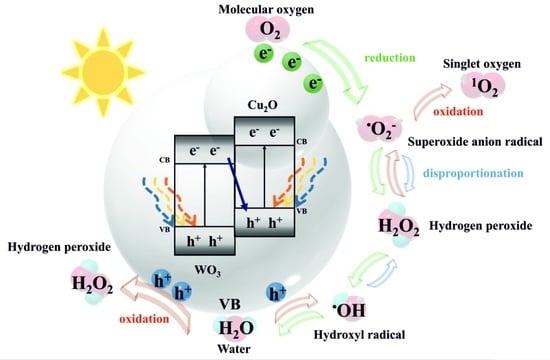
2.3.2. A Proposed Electron–Hole Transfer Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of WO3 Nanoplates
3.3. Synthesis of Cu2O/WO3 Photocatalyst
3.4. Photoreaction Apparatus, Procedure, and Analysis
3.5. Analytical Instruments and Procedures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Figures





References
- Solís, R.R.; Bedia, J.; Rodríguez, J.J.; Belver, C. A review on alkaline earth metal titanates for applications in photocatalytic water purification. Chem. Eng. J. 2021, 409, 128110. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst: (0 0 1) vs (1 0 1) facets of TiO2. Appl. Catal. B Environ. 2015, 164, 420–427. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Ji, L.; Jiang, X.; Fu, X. Preparation and characterization of direct Z-scheme photocatalyst Bi2O3/NaNbO3 and its reaction mechanism. Appl. Surf. Sci. 2014, 292, 357–366. [Google Scholar] [CrossRef]
- Ng, B.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Hitam, C.; Jalil, A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Han, C.; Yang, M.-Q.; Xu, Y.-J. Photocatalytic water splitting for solar hydrogen generation: Fundamentals and recent advancements. Int. Rev. Phys. Chem. 2016, 35, 1–36. [Google Scholar] [CrossRef]
- Chauhan, A.; Rastogi, M.; Scheier, P.; Bowen, C.; Kumar, R.V.; Vaish, R. Janus nanostructures for heterogeneous photocatalysis. Appl. Phys. Rev. 2018, 5, 041111. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Zhang, Y.; Cao, Y.; Luo, M.; Feng, Z.; Yang, W.; Liu, K.; Cao, H.; Yan, H. Pt@Cu2O/WO3 composite photocatalyst for enhanced photocatalytic water oxidation performance. Appl. Catal. B Environ. 2018, 237, 309–317. [Google Scholar] [CrossRef]
- Wan, J.; Du, X.; Liu, E.; Hu, Y.; Fan, J.; Hu, X. Z-scheme visible-light-driven Ag3PO4 nanoparticle@MoS2 quantum dot/few-layered MoS2 nanosheet heterostructures with high efficiency and stability for photocatalytic selective oxidation. J. Catal. 2017, 345, 281–294. [Google Scholar] [CrossRef]
- Jo, W.-K.; Natarajan, T.S. Facile Synthesis of Novel Redox-Mediator-free Direct Z-Scheme CaIn2S4 Marigold-Flower-like/TiO2 Photocatalysts with Superior Photocatalytic Efficiency. ACS Appl. Mater. Interfaces 2015, 7, 17138–17154. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a direct Z-scheme photocatalyst: Preparation and characterization of Bi2O3/g-C3N4 with high visible light activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef]
- Wei, S.; Ma, Y.; Chen, Y.; Liu, L.; Liu, Y.; Shao, Z. Fabrication of WO3/Cu2O composite films and their photocatalytic activity. J. Hazard. Mater. 2011, 194, 243–249. [Google Scholar] [CrossRef]
- Jo, W.-K.; Lee, J.Y.; Natarajan, T.S. Fabrication of hierarchically structured novel redox-mediator-free ZnIn2S4 marigold flower/Bi2WO6 flower-like direct Z-scheme nanocomposite photocatalysts with superior visible light photocatalytic efficiency. Phys. Chem. Chem. Phys. 2016, 18, 1000–1016. [Google Scholar] [CrossRef]
- Chen, S.; Ji, L.; Tang, W.; Fu, X. Fabrication, characterization and mechanism of a novel Z-scheme photocatalyst NaNbO3/WO3 with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 10759–10768. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Fang, X.; Zhai, T.; Gautam, U.K.; Li, L.; Wu, L.; Bando, Y.; Golberg, D. ZnS nanostructures: From synthesis to applications. Prog. Mater. Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Hao, J.; Qi, B.; Wei, J.; Li, D.; Zeng, F. A Z-scheme Cu2O/WO3 heterojunction for production of renewable hydrocarbon fuel from carbon dioxide. Fuel 2021, 287, 119439. [Google Scholar] [CrossRef]
- Toe, C.Y.; Scott, J.; Amal, R.; Ng, Y.H. Recent advances in suppressing the photocorrosion of cuprous oxide for photocatalytic and photoelectrochemical energy conversion. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 191–211. [Google Scholar] [CrossRef]
- Monllor-Satoca, D.; Borja, L.; Rodes, A.; Gómez, R.; Salvador, P. Photoelectrochemical Behavior of Nanostructured WO3 Thin-Film Electrodes: The Oxidation of Formic Acid. ChemPhysChem 2006, 7, 2540–2551. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Kim, C.W.; Pawar, A.U.; Kang, Y.S. Fabrication of p-Cu2O/n-Bi-WO3heterojunction thin films: Optical and photoelectrochemical properties. New J. Chem. 2016, 41, 755–762. [Google Scholar] [CrossRef]
- Minggu, L.J.; Ng, K.H.; Kadir, H.A.; Bin Kassim, M. Bilayer n-WO3/p-Cu2O photoelectrode with photocurrent enhancement in aqueous electrolyte photoelectrochemical reaction. Ceram. Int. 2014, 40, 16015–16021. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Liu, Z. Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting. Appl. Catal. B Environ. 2017, 201, 84–91. [Google Scholar] [CrossRef]
- Shi, W.; Guo, X.; Cui, C.; Jiang, K.; Li, Z.; Qu, L.; Wang, J.-C. Controllable synthesis of Cu2O decorated WO3 nanosheets with dominant (0 0 1) facets for photocatalytic CO2 reduction under visible-light irradiation. Appl. Catal. B Environ. 2019, 243, 236–242. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Wang, Y.; Li, Y.; Ma, D.; Zhao, Y.; Hou, S.; Hao, X. Oxalic acid mediated synthesis of WO3·H2O nanoplates and self-assembled nanoflowers under mild conditions. J. Solid State Chem. 2011, 184, 1661–1665. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Salama, T.M.; Hegazy, M.; Shahba, R.M.A.; Mohamed, S. Synthesis of hexagonal WO3 nanocrystals with various morphologies and their enhanced electrocatalytic activities toward hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 4724–4736. [Google Scholar] [CrossRef]
- Su, X.; Xiao, F.; Li, Y.; Jian, J.; Sun, Q.; Wang, J. Synthesis of uniform WO3 square nanoplates via an organic acid-assisted hydrothermal process. Mater. Lett. 2010, 64, 1232–1234. [Google Scholar] [CrossRef]
- Murau, P.C. Dissolution of Tungsten by Hydrogen Peroxide. Anal. Chem. 1961, 33, 1125–1126. [Google Scholar] [CrossRef]
- Kudo, T. A new heteropolyacid with carbon as a heteroatom in a Keggin-like structure. Nat. Cell Biol. 1984, 312, 537–538. [Google Scholar] [CrossRef]
- Okamoto, H.; Ishikawa, A.; Kudo, T. Amorphous and Crystalline Peroxopolytungstic Acids Formed from Tungsten and Hydrogen Peroxide. Bull. Chem. Soc. Jpn. 1989, 62, 2723–2724. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.C.; Choi, K.-S. Synthesis and characterization of high surface area CuWO4 and Bi2WO6 electrodes for use as photoanodes for solar water oxidation. J. Mater. Chem. A 2013, 1, 5006–5014. [Google Scholar] [CrossRef]
- Gillman, P.K. CNS toxicity involving methylene blue: The exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity. J. Psychopharmacol. 2010, 25, 429–436. [Google Scholar] [CrossRef]
- Pecquenard, B.; Castro-Garcia, S.; Livage, J.; Zavalij, P.Y.; Whittingham, M.S.; Thouvenot, R. Structure of Hydrated Tungsten Peroxides [WO2(O2)H2O]·nH2O. Chem. Mater. 1998, 10, 1882–1888. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Sharbatdaran, M.; Novinrooz, A.; Noorkojouri, H. Preparation and characterization of WO3 electrochromic films obtained by the sol-gel process, Iran. J. Chem. Chem. Eng. 2006, 25, 25–29. [Google Scholar]
- Boulova, M.; Lucazeau, G. Crystallite Nanosize Effect on the Structural Transitions of WO3 Studied by Raman Spectroscopy. J. Solid State Chem. 2002, 167, 425–434. [Google Scholar] [CrossRef]
- Su, C.-Y.; Lin, H.-C.; Lin, C.-K. Fabrication and optical properties of Ti-doped W18O49 nanorods using a modified plasma-arc gas-condensation technique. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2009, 27, 2170. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, A.Y.; Nishimoto, S.-I. Photocatalytic Activity of Amorphous−Anatase Mixture of Titanium(IV) Oxide Particles Suspended in Aqueous Solutions. J. Phys. Chem. B 1997, 101, 3746–3752. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Nogami, K.; Tanaka, M.; Ohtani, B. Correlation between Surface Area and Photocatalytic Activity for Acetaldehyde Decomposition over Bismuth Tungstate Particles with a Hierarchical Structure. Langmuir 2010, 26, 7174–7180. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Kuritka, I.; Masar, M.; Machovsky, M.; Urbanek, P.; Bazant, P.; Janota, P.; Dvorackova, M. Contributions of morphological and structural parameters at different hierarchical morphology levels to photocatalytic activity of mesoporous nanostructured ZnO. Appl. Surf. Sci. 2020, 513, 145773. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Schevciw, O.; White, W.B. The optical absorption edge of rare earth sesquisulfides and alkaline earth—Rare earth sulfides. Mater. Res. Bull. 1983, 18, 1059–1068. [Google Scholar] [CrossRef]
- Köferstein, R.; Jäger, L.; Ebbinghaus, S.G. Magnetic and optical investigations on LaFeO3 powders with different particle sizes and corresponding ceramics. Solid State Ion. 2013, 249–250, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Nagy, D.D.; Szilágyi, I.M.; Fan, X. Effect of the morphology and phases of WO3 nanocrystals on their photocatalytic efficiency. RSC Adv. 2016, 6, 33743–33754. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Khiavi, N.D.; Katal, R.; Eshkalak, S.K.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible Light Driven Heterojunction Photocatalyst of CuO–Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Adachi, S. Properties of Group-IV, III-V and II-VI Semiconductors. In Wiley Series in Materials for Electronic and Optoelectronic Applications; John Wiley & Sons Ltd: Chichester, UK, 2005. [Google Scholar]
- Butler, M.A.; Ginley, D.S. Prediction of Flatband Potentials at Semiconductor-Electrolyte Interfaces from Atomic Electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J. NIST Atomic Spectra Database Team, NIST Atomic Spectra Database (Version 5.8); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020. [CrossRef]
- Bartmess, J.E. Negative Ion Energetics Data. In NIST Chemistry WebBook, NIST Stand. Ref. Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2021; p. 20899. [Google Scholar]
- Mulliken, R.S. A New Electroaffinity Scale; Together with Data on Valence States and on Valence Ionization Potentials and Electron Affinities. J. Chem. Phys. 1934, 2, 782–793. [Google Scholar] [CrossRef]
- Stevanović, V.; Lany, S.; Ginley, D.S.; Tumas, W.; Zunger, A. Assessing capability of semiconductors to split water using ionization potentials and electron affinities only. Phys. Chem. Chem. Phys. 2014, 16, 3706–3714. [Google Scholar] [CrossRef]
- Barbero, N.; Vione, D. Why Dyes Should Not Be Used to Test the Photocatalytic Activity of Semiconductor Oxides. Environ. Sci. Technol. 2016, 50, 2130–2131. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Photobleaching of methylene blue sensitised by TiO2: An ambiguous system? J. Photochem. Photobiol. A Chem. 1999, 127, 123–134. [Google Scholar] [CrossRef]
- Houas, A. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- International Organization for Standardization. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue; International Standard Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Mills, A.; Hill, C.; Robertson, P.K. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. UV LED Sources for Heterogeneous Photocatalysis; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 159–179. [Google Scholar]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Wood, P.M. The potential diagram for oxygen at pH 7. Biochem. J. 1988, 253, 287–289. [Google Scholar] [CrossRef] [PubMed]
- De Tacconi, N.R.; Carmona, J.; Rajeshwar, K. Reversibility of Photoelectrochromism at the TiO2/Methylene Blue Interface. J. Electrochem. Soc. 1997, 144, 2486–2490. [Google Scholar] [CrossRef]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]















| Pure WO3 | Cu2O(1.0 wt %)/WO3 | Cu2O(5.0 wt %)/WO3 | Cu2O(10.0 wt %)/WO3 | |
|---|---|---|---|---|
| Rate constant k × 10−3 min−1; LED with wavelength 377 nm | ||||
| No scavenger | 0.9 ± 0.06 | 2.1 ± 0.05 | 2.4 ± 0.09 | 3.1 ± 0.06 |
| EDTA | 0.2 ± 0.01 | 1.4 ± 0.08 | 2.2 ± 0.07 | 3.4 ± 0.14 |
| t-butOH | 1.4 ± 0.10 | 1.6 ± 0.06 | 1.5 ± 0.06 | 1.5 ± 0.07 |
| PBQ | 1.8 ± 0.06 | 3.2 ± 0.30 | 2.8 ± 0.10 | 1.9 ± 0.08 |
| Rate constant k × 10−3 min−1; LED with wavelength 429 nm | ||||
| No scavenger | 1.5 ± 0.06 | 1.9 ± 0.04 | 1.9 ± 0.03 | 2.6 ± 0.06 |
| EDTA | 0.3 ± 0.02 | 1.1 ± 0.03 | 2.0 ± 0.11 | 2.8 ± 0.17 |
| t-butOH | 1.6 ± 0.05 | 1.9 ± 0.17 | 1.8 ± 0.07 | 1.7 ± 0.02 |
| PBQ | 0.5 ± 0.08 | 2.4 ± 0.10 | 2.5 ± 0.15 | 2.8 ± 0.26 |
| Rate constant k × 10−3 min−1; LED with wavelength 523 nm | ||||
| No scavenger | 0.1 ± 0.01 | 0.6 ± 0.03 | 0.3 ± 0.02 | 0.3 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.; Guler, A.C.; Masar, M.; Urbanek, P.; Urbanek, M.; Skoda, D.; Suly, P.; Machovsky, M.; Galusek, D.; Kuritka, I. Solid-State Synthesis of Direct Z-Scheme Cu2O/WO3 Nanocomposites with Enhanced Visible-Light Photocatalytic Performance. Catalysts 2021, 11, 293. https://doi.org/10.3390/catal11020293
Ali H, Guler AC, Masar M, Urbanek P, Urbanek M, Skoda D, Suly P, Machovsky M, Galusek D, Kuritka I. Solid-State Synthesis of Direct Z-Scheme Cu2O/WO3 Nanocomposites with Enhanced Visible-Light Photocatalytic Performance. Catalysts. 2021; 11(2):293. https://doi.org/10.3390/catal11020293
Chicago/Turabian StyleAli, Hassan, Ali Can Guler, Milan Masar, Pavel Urbanek, Michal Urbanek, David Skoda, Pavol Suly, Michal Machovsky, Dusan Galusek, and Ivo Kuritka. 2021. "Solid-State Synthesis of Direct Z-Scheme Cu2O/WO3 Nanocomposites with Enhanced Visible-Light Photocatalytic Performance" Catalysts 11, no. 2: 293. https://doi.org/10.3390/catal11020293
APA StyleAli, H., Guler, A. C., Masar, M., Urbanek, P., Urbanek, M., Skoda, D., Suly, P., Machovsky, M., Galusek, D., & Kuritka, I. (2021). Solid-State Synthesis of Direct Z-Scheme Cu2O/WO3 Nanocomposites with Enhanced Visible-Light Photocatalytic Performance. Catalysts, 11(2), 293. https://doi.org/10.3390/catal11020293