Enhancement of Pentachlorophenol Removal in a Historically Contaminated Soil by Adding Ascorbic Acid to H2O2/Magnetite System
Abstract
:1. Introduction
2. Results and Discussion
2.1. PCP Remediation Efficiency in Soil Slurry Experiments
2.2. Combined Treatments in Batch Slurry Experiments
2.3. Dissolved and Structural Fe(II) Analyses
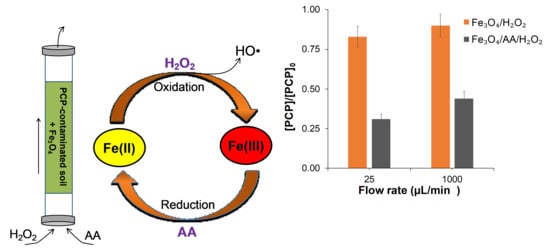
2.4. PCP Remediation in Dynamic Column Experiments
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Oxidation Experiments
4.3. Extraction and Analysis of PCP
4.4. Mössbauer Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Usman, M.; Ho, Y.-S. A bibliometric study of the Fenton oxidation for soil and water remediation. J. Environ. Manag. 2020, 270, 110886. [Google Scholar] [CrossRef]
- Koppenol, W.H. The centennial of the Fenton reaction. Free Radic. Biol. Med. 1993, 15, 645–651. [Google Scholar] [CrossRef]
- Usman, M.; Hanna, K.; Haderlein, S. Fenton Oxidation to Remediate PAHs in Contaminated Soils: A Critical Review of Major Limitations and Counter-Strategies. Sci. Total Environ. 2016, 569–570, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.; Hanna, K.; Chiron, S. Fenton-Like Oxidation of 2,4,6-Trinitrotoluene Using Different Iron Minerals. Sci. Total Environ. 2007, 385, 242–251. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, J.M.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Hanna, K.; Faure, P. Remediation of oil-contaminated harbor sediments by chemical oxidation. Sci. Total Environ. 2018, 634, 1100–1107. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Wei, X.; Wu, H.; Sun, F. Magnetite/Fe-Al-montmorillonite as a Fenton catalyst with efficient degradation of phenol. J. Colloid Interface Sci. 2017, 504, 611–619. [Google Scholar] [CrossRef]
- Munoz, M.; Domínguez, P.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Naturally-Occurring Iron Minerals as Inexpensive Catalysts for CWPO. Appl. Catal. B 2017, 203, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Ai, Z.; Zhao, J.; Zhang, L. Ascorbic acid/Fe@Fe2O3: A highly efficient combined Fenton reagent to remove organic contaminants. J. Hazard. Mater. 2016, 310, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Xie, G.; He, D.; Zhang, L. Ascorbic acid promoted magnetite Fenton degradation of alachlor: Mechanistic insights and kinetic modeling. Appl. Catal. B Environ. 2020, 267C, 118383. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Jia, F.; Song, F.; Zhao, J.; Zhang, L. Ascorbate-Promoted Surface Iron Cycle for Efficient Heterogeneous Fenton Alachlor Degradation with Hematite Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 8751–8758. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, H.; Wang, J.; Ai, J. Rapid and continuous oxidation of organic contaminants with ascorbic acid and a modified ferric/persulfate system. Chem. Eng. J. 2015, 270, 73–79. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Li, M.; Zhang, Y.; Yuan, S.; Ai, Z.; Zhao, J.; Zhang, L. Fenton oxidation of organic contaminants with aquifer sediment activated by ascorbic acid. Chem. Eng. J. 2018, 348, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Usman, M.; Faure, P.; Lorgeoux, C.; Ruby, C.; Hanna, K. Treatment of hydrocarbon contamination under flow through conditions by using magnetite catalyzed chemical oxidation. Env. Sci. Pollut. Res. 2013, 20, 22–30. [Google Scholar] [CrossRef]
- Rybnikova, V.; Singhal, N.; Hanna, K. Remediation of an aged PCP-contaminated soil by chemical oxidation under flow-through conditions. Chem. Eng. J. 2017, 314, 202–211. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Pentachlorophenol and Some Related Compounds. Some Ind. Chem. 1994, 60, 389–433. [Google Scholar]
- Usman, M.; Tascone, O.; Faure, P.; Hanna, K. Chemical oxidation of hexachlorocyclohexanes (HCHs) in contaminated soils. Sci. Total Environ. 2014, 476–477, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Faure, P.; Ruby, C.; Hanna, K. Remediation of PAH-contaminated soils by magnetite catalyzed Fenton-like oxidation. Appl. Catal. B 2012, 117–118, 10–17. [Google Scholar] [CrossRef]
- Usman, M.; Tascone, O.; Rybnikova, V.; Faure, P.; Hanna, K. Application of chemical oxidation to remediate HCH-contaminated soil under batch and flow through conditions. Env. Sci. Pollut. Res. 2017, 24, 14748–14757. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Hou, Y.; Zhang, E.; Tu, S.; Xiong, S. Ascorbic acid induced activation of persulfate for pentachlorophenol degradation. Chemosphere 2019, 229, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Liu, H.; Ma, J. Ascorbic acid/Fe0 composites as an effective persulfate activator for improving the degradation of rhodamine B. RSC Adv. 2018, 8, 12791–12798. [Google Scholar] [CrossRef] [Green Version]
- Hanna, K.; Chiron, S.; Oturan, M.A. Coupling enhanced water solubilization with cyclodextrin to indirect electrochemical treatment for pentachlorophenol contaminated soil remediation. Water Res. 2005, 39, 2763–2773. [Google Scholar] [CrossRef]
- Gorski, C.A.; Scherer, M.M. Determination of nanoparticulate magnetite stoichiometry by Mössbauer spectroscopy, acidic dissolution, and powder X-ray diffraction: A critical review. Am. Mineral. 2010, 95, 1017–1026. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, H.; Xue, X. Ultrasound Enhanced Heterogeneous Activation of Peroxydisulfate by Magnetite Catalyst for the Degradation of Tetracycline in Water. Sep. Purif. Technol. 2012, 84, 147–152. [Google Scholar] [CrossRef]
- Monfort, O.; Usman, M.; Hanna, K. Ferrate (VI) oxidation of pentachlorophenol in water and soil. Chemosphere 2020, 253, 126550. [Google Scholar] [CrossRef]
- Bertinetti, S.; Hanna, K.; Minella, M.; Minero, C.; Vione, D. Fenton-type processes triggered by titanomagnetite for the degradation of phenol as model pollutant. Desalin. Water Treat. 2019, 151, 117–127. [Google Scholar] [CrossRef]
- Rancourt, D.G.; Ping, J.Y. Voigt-based methods for arbitrary-shape static hyperfine parameter distributions in Mössbauer spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B 1991, 58, 85–97. [Google Scholar] [CrossRef]







| System | Purpose of Experiment | Reagent Doses | Reaction Conditions | |||
|---|---|---|---|---|---|---|
| Treatment | Fe3O4 (g kg−1 Soil w/w) | Ascorbic Acid (mM) | H2O2 (mM) | |||
| Real soil (batch study) | To evaluate the impact of amounts of oxidant, AA, and Fe3O4. | Fe3O4 | 5 | - | - | PCP = 6 mg kg−1 (real soil), pH = 7.2 (not adjusted), Volume of solution = 2 mL Reaction time = 24 h |
| Fe3O4 + AA | 5 | 125 | ||||
| Fe3O4 + H2O2 | 5 | - | 200 | |||
| Fe3O4 + H2O2 | 5 | - | 500 | |||
| Fe3O4 + AA+ H2O2 | 5 | 50 | 200 | |||
| Fe3O4 + AA+ H2O2 | 5 | 125 | 500 | |||
| Fe3O4 | 25 | - | - | |||
| Fe3O4 + AA | 25 | 125 | ||||
| Fe3O4 + H2O2 | 25 | - | 200 | |||
| Fe3O4 + H2O2 | 25 | - | 500 | |||
| Fe3O4 + AA+ H2O2 | 25 | 50 | 200 | |||
| Fe3O4 + AA+ H2O2 | 25 | 125 | 500 | |||
| To evaluate the degradation of PCP at different time points | Fe3O4 | 5 | - | - | ||
| Fe3O4 + AA+ H2O2 | 5 | 50 | 200 | |||
| Real soil (column study) | To evaluate the impact of reagent doses and flow rate Following flow rates were chosen: 0.025, 0.1, 0.5, and 1 mL min−1 | Fe3O4 + AA | 5 | 10 | - | PCP = 6 mg kg−1 (real soil), pH = 7.2 (not adjusted), Volume of the injected solution = 500 mL |
| Fe3O4 + H2O2 | 5 | - | 10 | |||
| Fe3O4 + AA+ H2O2 | 5 | 5 | 10 | |||
| Fe3O4 + AA+ H2O2 | 5 | 10 | 10 | |||
| Treatment | Fe3O4 (g L−1) | Ascorbic acid (mM) | H2O2 (mM) | |||
| Aqueous solution | To evaluate the treatment efficiency of different oxidation systems at different time points | Fe3O4 | 0.5 | - | - | PCP = 20 mg L−1, pH = 7.2, Volume of solution = 100 mL, Reaction time = 300 min |
| AA | - | 4 | - | |||
| H2O2 | - | - | 4 | |||
| Fe3O4 + AA | 0.5 | 4 | - | |||
| Fe3O4 + H2O2 | 0.5 | - | 4 | |||
| AA + H2O2 | - | 4 | 4 | |||
| Fe3O4 + AA+ H2O2 | 0.5 | 4 | 4 | |||
| To evaluate the impact of AA/H2O2 ratio on treatment efficiency at different time points | Fe3O4 + AA+ H2O2 | 0.5 | 4 | 4 | ||
| Fe3O4 + AA+ H2O2 | 0.5 | 2 | 2 | |||
| Fe3O4 + AA+ H2O2 | 0.5 | 4 | 2 | |||
| Fe3O4 + AA+ H2O2 | 0.5 | 4 | 8 | |||
| Radical | Species and Concentrations | k″ (M−1 s−1) | k′ (s−1) | Percent of Reactivity |
|---|---|---|---|---|
| HO• | PCP (75 µM) | 3.6 × 109 [27] | 2.7 × 105 | 0.7 |
| AA (4 mM) | 1.0 × 1010 [13] | 4.0 × 107 | 99.0 | |
| H2O2 (4 mM) | 3.0 × 107 [13] | 1.2 × 105 | 0.3 |
| CS | e | H | stdev(H) | R.A. | Error | χ2 | Fe(II)/Fe(III) | ||
|---|---|---|---|---|---|---|---|---|---|
| (mm/s) | (mm/s) | (T) | (T) | % | |||||
| Magnetite (before treatment) | MagT | 0.39 | −0.02 | 51.3 | 1.1 | 58.4 | 1.1 | 2.8 | 0.26 |
| MagO | 0.73 | −0.01 | 48.0 | 3.2 | 41.6 | 1.1 | - | - | |
| Magnetite (after treatment) | MagT | 0.38 | −0.02 | 51.1 | 1.0 | 61.3 | 2.2 | 1.1 | 0.24 |
| MagO | 0.77 | −0.02 | 48.2 | 2.6 | 38.7 | 2.2 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, M.; Monfort, O.; Haderlein, S.; Hanna, K. Enhancement of Pentachlorophenol Removal in a Historically Contaminated Soil by Adding Ascorbic Acid to H2O2/Magnetite System. Catalysts 2021, 11, 331. https://doi.org/10.3390/catal11030331
Usman M, Monfort O, Haderlein S, Hanna K. Enhancement of Pentachlorophenol Removal in a Historically Contaminated Soil by Adding Ascorbic Acid to H2O2/Magnetite System. Catalysts. 2021; 11(3):331. https://doi.org/10.3390/catal11030331
Chicago/Turabian StyleUsman, Muhammad, Olivier Monfort, Stefan Haderlein, and Khalil Hanna. 2021. "Enhancement of Pentachlorophenol Removal in a Historically Contaminated Soil by Adding Ascorbic Acid to H2O2/Magnetite System" Catalysts 11, no. 3: 331. https://doi.org/10.3390/catal11030331
APA StyleUsman, M., Monfort, O., Haderlein, S., & Hanna, K. (2021). Enhancement of Pentachlorophenol Removal in a Historically Contaminated Soil by Adding Ascorbic Acid to H2O2/Magnetite System. Catalysts, 11(3), 331. https://doi.org/10.3390/catal11030331