Hydrothermal Sintering and Oxidation of an Alumina-Supported Nickel Methanation Catalyst Studied Using In Situ Magnetometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ex Situ PXRD and TEM
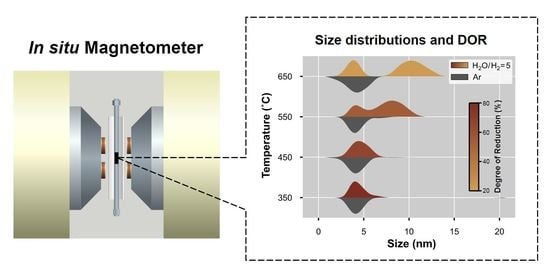
2.2. In Situ Magnetometry
2.2.1. Degree of Reduction
2.2.2. Magnetometry-Based Size Analysis
2.2.3. Methods Used with the Langevin Equation
2.2.4. Derivation of Size Distributions from Magnetometry Data
2.2.5. Comparison of the Size Analysis from the TEM and Magnetometry Studies
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Ex Situ Catalyst Characterisation
3.3. Magnetism and Magnetometry
3.3.1. In Situ Reduction Studies in a Hydrogen Atmosphere
3.3.2. In Situ Sintering Studies in an Inert Atmosphere
3.3.3. In Situ Sintering and Oxidation Studies in a Steam/Hydrogen Atmosphere
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Quan, Y.; Hao, P.; Zhao, J.; Ren, J. Highly anti-sintering and anti-coking ordered mesoporous silica carbide supported nickel catalyst for high temperature CO methanation. Fuel 2019, 257, 116006. [Google Scholar] [CrossRef]
- Kiendl, I.; Klemm, M.; Clemens, A.; Herrman, A. Dilute gas methanation of synthesis gas from biomass gasification. Fuel 2014, 123, 211–217. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Seemann, M.C.; Moergeli, R.; Biollaz, S.M.A.; Schildhauer, T.J. Synthetic natural gas from wood: Reactions of ethylene in fluidised bed methanation. Appl. Catal. A Gen. 2013, 462–463, 150–156. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Zhang, S. Coke oven gas: Availability, properties, purification, and utilization in China. Fuel 2013, 113, 287–299. [Google Scholar] [CrossRef]
- Gong, D.; Li, S.; Guo, S.; Tang, H.; Wang, H.; Liu, Y. Lanthanum and cerium co-modified Ni/SiO2 catalyst for CO methanation from syngas. Appl. Surf. Sci. 2018, 434, 351–364. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. Rsc Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Vannice, M.A. The catalytic synthesis of hydrocarbons from H2CO mixtures over the group VIII metals: I. The specific activities and product distributions of supported metals. J. Catal. 1975, 37, 449–461. [Google Scholar] [CrossRef]
- Foppa, L.; Iannuzzi, M.; Copéret, C.; Comas-Vives, A. CO methanation on ruthenium flat and stepped surfaces: Key role of H-transfers and entropy revealed by ab initio molecular dynamics. J. Catal. 2019, 371, 270–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Liu, Q.; Bian, B. MoOx-Doped Ordered Mesoporous Ni/Al2O3 Catalyst for CO Methanation. Energy Technol. 2020, 8, 2000165. [Google Scholar] [CrossRef]
- Ren, J.; Li, H.; Jin, Y.; Zhu, J.; Liu, S.; Lin, J.; Li, Z. Silica/titania composite-supported Ni catalysts for CO methanation: Effects of Ti species on the activity, anti-sintering, and anti-coking properties. Appl. Catal. B Environ. 2017, 201, 561–572. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, P.; Zhang, Z.; Zhou, Y.; Zhang, J.; Zhang, H.; Shi, Z.; Han, R.P.S.; Huang, F.; Ma, D. Nickel catalyst stabilization via graphene encapsulation for enhanced methanation reaction. J. Catal. 2016, 334, 42–51. [Google Scholar] [CrossRef]
- Qin, Z.; Ren, J.; Miao, M.; Li, Z.; Lin, J.; Xie, K. The catalytic methanation of coke oven gas over Ni-Ce/Al2O3 catalysts prepared by microwave heating: Effect of amorphous NiO formation. Appl. Catal. B Environ. 2015, 164, 18–30. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Moulijn, J.A.; van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: Is it predictable?: What to do? Appl. Catal. A Gen. 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Claeys, M.; Dry, M.E.; van Steen, E.; van Berge, P.J.; Booyens, S.; Crous, R.; van Helden, P.; Labuschagne, J.; Moodley, D.J.; Saib, A.M. Impact of Process Conditions on the Sintering Behavior of an Alumina-Supported Cobalt Fischer–Tropsch Catalyst Studied with an in Situ Magnetometer. Acs Catal. 2015, 5, 841–852. [Google Scholar] [CrossRef]
- Thomson, W. On the equilibrium of vapour at a curved surface of liquid. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1871, 42, 448–452. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, F.; Zhong, Z.; Xu, G.; Su, F. Anti-sintering ZrO2-modified Ni/α-Al2O3 catalyst for CO methanation. Rsc Adv. 2016, 6, 20979–20986. [Google Scholar] [CrossRef]
- Lucchini, M.A.; Testino, A.; Kambolis, A.; Proff, C.; Ludwig, C. Sintering and coking resistant core–shell microporous silica–nickel nanoparticles for CO methanation: Towards advanced catalysts production. Appl. Catal. B Environ. 2016, 182, 94–101. [Google Scholar] [CrossRef]
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Agnelli, M.; Swaan, H.M.; Marquez-Alvarez, C.; Martin, G.A.; Mirodatos, C. CO Hydrogenation on a Nickel Catalyst: II. A Mechanistic Study by Transient Kinetics and Infrared Spectroscopy. J. Catal. 1998, 175, 117–128. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Jiang, J.; Jin, G.; Li, H.; Gu, F.; Xu, G.; Zhong, Z.; Su, F. SiO2-stabilized Ni/t-ZrO2 catalysts with ordered mesopores: One-pot synthesis and their superior catalytic performance in CO methanation. Catal. Sci. Technol. 2016, 6, 3529–3543. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Catalytic steam reforming. In Catalysis: Science and Technology, 1st ed.; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin, Heidelberg, 1984; pp. 1–117. [Google Scholar]
- Perego, C.; Villa, P. Catalyst preparation methods. Catal. Today 1997, 34, 281–305. [Google Scholar] [CrossRef]
- Reuel, R.C.; Bartholomew, C.H. Effects of support and dispersion on the CO hydrogenation activity/selectivity properties of cobalt. J. Catal. 1984, 85, 78–88. [Google Scholar] [CrossRef]
- van Steen, E.; Claeys, M.; Dry, M.E.; van de Loosdrecht, J.; Viljoen, E.L.; Visagie, J.L. Stability of nanocrystals: Thermodynamic analysis of oxidation and re-reduction of cobalt in water/hydrogen mixtures. J. Phys. Chem. B 2005, 109, 3575–3577. [Google Scholar] [CrossRef]
- Sehested, J.; Gelten, J.A.; Helveg, S. Sintering of nickel catalysts: Effects of time, atmosphere, temperature, nickel-carrier interactions, and dopants. Appl. Catal. A Gen. 2006, 309, 237–246. [Google Scholar] [CrossRef]
- Champon, I.; Bengaouer, A.; Chaise, A.; Thomas, S.; Roger, A.-C. Modelling the Sintering of Nickel Particles Supported on γ-Alumina under Hydrothermal Conditions. Catalysts 2020, 10, 1477. [Google Scholar] [CrossRef]
- Wolf, M.; Fischer, N.; Claeys, M. Water-induced deactivation of cobalt-based Fischer–Tropsch catalysts. Nat. Catal. 2020, 3, 962–965. [Google Scholar] [CrossRef]
- Wolf, M.; Fischer, N.; Claeys, M. Capturing the interconnectivity of water-induced oxidation and sintering of cobalt nanoparticles during the Fischer-Tropsch synthesis in situ. J. Catal. 2019, 374, 199–207. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Fischer, N.; Claeys, M. In situ characterization of Fischer–Tropsch catalysts: A review. J. Phys. D Appl. Phys. 2020, 53, 293001. [Google Scholar] [CrossRef]
- Knacke, O.; Kubaschewski, O.; Hesselmann, K. Thermochemical Properties of Inorganic Substances, 2nd ed.; Springer: Berlin, Germany, 1991. [Google Scholar]
- Wolf, M.; Kotze, H.; Fischer, N.; Claeys, M. Size dependent stability of cobalt nanoparticles on silica under high conversion Fischer-Tropsch environment. Faraday Discuss. 2017, 197, 243–268. [Google Scholar] [CrossRef]
- Nyathi, T.M.; Fischer, N.; York, A.P.E.; Morgan, D.J.; Hutchings, G.J.; Gibson, E.K.; Wells, P.P.; Catlow, C.R.A.; Claeys, M. Impact of Nanoparticle–Support Interactions in Co3O4/Al2O3 Catalysts for the Preferential Oxidation of Carbon Monoxide. Acs Catal. 2019, 9, 7166–7178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmon, J. Magnetic measurements and catalysis. In Catalyst Characterization: Physical Techniques for Solid Materials, 1st ed.; Imelik, B., Vedrine, J.C., Eds.; Springer: New York, NY, USA, 1994; pp. 585–607. [Google Scholar]
- Ishizaki, T.; Yatsugi, K.; Akedo, K. Effect of Particle Size on the Magnetic Properties of Ni Nanoparticles Synthesized with Trioctylphosphine as the Capping Agent. Nanomaterials 2016, 6, 172. [Google Scholar] [CrossRef]
- Moodley, D.; Claeys, M.; van Steen, E.; van Helden, P.; Kistamurthy, D.; Weststrate, K.-J.; Niemantsverdriet, H.; Saib, A.; Erasmus, W.; van de Loosdrecht, J. Sintering of cobalt during FTS: Insights from industrial and model systems. Catal. Today 2020, 342, 59–70. [Google Scholar] [CrossRef]
- Wolf, M.; Gibson, E.K.; Olivier, E.J.; Neethling, J.H.; Catlow, C.R.A.; Fischer, N.; Claeys, M. Water-Induced Formation of Cobalt-Support Compounds under Simulated High Conversion Fischer–Tropsch Environment. Acs Catal. 2019, 9, 4902–4918. [Google Scholar] [CrossRef]
- Sehested, J.; Larsen, N.W.; Falsig, H.; Hinnemann, B. Sintering of nickel steam reforming catalysts: Effective mass diffusion constant for Ni-OH at nickel surfaces. Catal. Today 2014, 228, 22–31. [Google Scholar] [CrossRef]
- Sehested, J. Sintering of nickel steam-reforming catalysts: Effects of temperature and steam and hydrogen pressures. J. Catal. 2004, 223, 432–443. [Google Scholar] [CrossRef]
- Rasmussen, F.B.; Sehested, J.; Teunissen, H.T.; Molenbroek, A.M.; Clausen, B.S. Sintering of Ni/Al2O3 catalysts studied by anomalous small angle X-ray scattering. Appl. Catal. A Gen. 2004, 267, 165–173. [Google Scholar] [CrossRef]
- Sehested, J. Sintering of nickel steam-reforming catalysts. J. Catal. 2003, 217, 417–426. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Sorensen, W.L. Sintering kinetics of silica-and alumina-supported nickel in hydrogen atmosphere. J. Catal. 1983, 81, 131–141. [Google Scholar] [CrossRef]
- Prieto, G.; Zečević, J.; Friedrich, H.; de Jong, K.P.; de Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2013, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.R.; Delariva, A.T.; Hansen, T.W.; Helveg, S.; Sehested, J.; Hansen, P.L.; Garzon, F.; Datye, A.K. Relating rates of catalyst sintering to the disappearance of individual nanoparticles during Ostwald ripening. J. Am. Chem. Soc. 2011, 133, 20672–20675. [Google Scholar] [CrossRef] [PubMed]
- DeLaRiva, A.T.; Hansen, T.W.; Challa, S.R.; Datye, A.K. In situ transmission electron microscopy of catalyst sintering. J. Catal. 2013, 308, 291–305. [Google Scholar] [CrossRef] [Green Version]
- Bellare, A.; Dadyburjor, D.B.; Kelley, M.J. Evolution of bimodal distributions in the sintering of model supported metal catalysts. J. Catal. 1989, 117, 78–90. [Google Scholar] [CrossRef]
- Kolb, M.; Agnelli, M.; Mirodatos, C. Kinetics of Bimodal Grain Size Distribution of a Ni Catalyst During Hydrogenation of CO. In Studies in Surface Science and Catalysis; Guczi, L., Solymosi, F., TÉTÉNyi, P., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 1993; Volume 75, pp. 2833–2836. [Google Scholar]
- Bartholomew, C.H.; Pannell, R.B. The stoichiometry of hydrogen and carbon monoxide chemisorption on alumina- and silica-supported nickel. J. Catal. 1980, 65, 390–401. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P. Particle Size and Dispersion Measurements. In Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 738–765. [Google Scholar]
- Claeys, M.C.M.; van Steen, E.W.J.; Visagie, J.L.; van de Loosdrecht, J. Magnetometer. U.S. Patent 8773118B2, 8 July 2014. [Google Scholar]
- Fischer, N.; Clapham, B.; Feltes, T.; van Steen, E.; Claeys, M. Size-Dependent Phase Transformation of Catalytically Active Nanoparticles Captured In Situ. Angew. Chem. Int. Ed. 2014, 53, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Crangle, J.; Goodman, G.M.; Sucksmith, W. The magnetization of pure iron and nickel. Proc. R. Soc. Lond. A Math. Phys. Sci. 1971, 321, 477–491. [Google Scholar] [CrossRef]
- Shahzad, F.; Nadeem, K.; Weber, J.; Krenn, H.; Knoll, P. Magnetic behavior of NiO nanoparticles determined by SQUID magnetometry. Mater. Res. Express 2017, 4, 086102. [Google Scholar] [CrossRef]
- Kraft, D. A Software Package for Sequential Quadratic Programming; DLR German Aerospace Center-Institute for Flight Mechanics: Koln, Germany, 1988. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Condition | dn, TEM (nm) |
|---|---|
| Post-reduction at 500 °C, atmospheric pressure | 2.7 ± 1.1 |
| Post-exposure to Ar at 650 °C, atmospheric pressure | 4.0 ± 1.1 |
| Post-exposure to PH2O/PH2 = 5 at 650 °C, 10 bar | 18.9 ± 15.3 |
| Temperature (°C) | Degree of Reduction (%) | |
|---|---|---|
| Ar | H2O/H2 = 5 | |
| 350 | 86.2 | 80.8 |
| 450 | 85.6 | 67.4 |
| 550 | 82.8 | 54.0 |
| 650 | - * | 22.2 |
| Temperature (°C) | ||
|---|---|---|
| Ar | H2O/H2 = 5 | |
| 350 | 2.8 | 0.9 |
| 450 | 1.8 | 1.5 |
| 550 | 3.4 | 2.2 |
| 650 | 1.8 | 8.4 |
| Temperature (°C) | dv, mag | |
|---|---|---|
| Ar | H2O/H2 = 5 | |
| 350 | 4.1 ± 0.8 | 4.2 ± 0.7 |
| 450 | 4.1 ± 0.9 | 4.6 ± 1.6 |
| 550 | 4.1 ± 1.2 | 7.4 ± 2.2 |
| 650 | 4.2 ± 3.1 | 9.3 ± 2.9 |
| Condition | dv, TEM (nm) | dv, mag (nm) |
|---|---|---|
| Post-reduction at 500 °C, atmospheric pressure | 3.8 ± 1.0 | 4.0 ± 0.8 |
| Post-exposure to Ar at 650 °C, atmospheric pressure | 4.9 ± 1.1 | 4.2 ± 3.1 |
| Post-exposure to PH2O/PH2 = 5 at 650 °C, 10 bar | 49.7 ± 14.8 | 9.3 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maphutha, M.; de Oliveira, D.; Nyathi, T.M.; Fadlalla, M.I.; Henkel, R.; Fischer, N.; Claeys, M. Hydrothermal Sintering and Oxidation of an Alumina-Supported Nickel Methanation Catalyst Studied Using In Situ Magnetometry. Catalysts 2021, 11, 636. https://doi.org/10.3390/catal11050636
Maphutha M, de Oliveira D, Nyathi TM, Fadlalla MI, Henkel R, Fischer N, Claeys M. Hydrothermal Sintering and Oxidation of an Alumina-Supported Nickel Methanation Catalyst Studied Using In Situ Magnetometry. Catalysts. 2021; 11(5):636. https://doi.org/10.3390/catal11050636
Chicago/Turabian StyleMaphutha, Malebelo, Dominic de Oliveira, Thulani M. Nyathi, Mohamed I. Fadlalla, Robert Henkel, Nico Fischer, and Michael Claeys. 2021. "Hydrothermal Sintering and Oxidation of an Alumina-Supported Nickel Methanation Catalyst Studied Using In Situ Magnetometry" Catalysts 11, no. 5: 636. https://doi.org/10.3390/catal11050636
APA StyleMaphutha, M., de Oliveira, D., Nyathi, T. M., Fadlalla, M. I., Henkel, R., Fischer, N., & Claeys, M. (2021). Hydrothermal Sintering and Oxidation of an Alumina-Supported Nickel Methanation Catalyst Studied Using In Situ Magnetometry. Catalysts, 11(5), 636. https://doi.org/10.3390/catal11050636