2.1. Effect of the Pd Precursor on the Structural Properties of Pd/SiO2 Catalyst
Table 1 gives the basic structural properties of Pd/SiO
2 catalysts prepared by the dry ball-milling method with different Pd precursors. Obviously, all the Pd/SiO
2 catalysts display a similar surface area rather close to that of the SiO
2 support, suggesting that such a mechanical milling mixing method has little impact on the textural structure of the pristine SiO
2 support. In addition, the actual Pd loading of all the Pd/SiO
2 catalysts measured by ICP-OES is approximately equal to the designated value, proving that it is able to avoid any material loss by using the mechanical mixing method to prepare the Pd/SiO
2 catalysts. In contrast, four Pd/SiO
2 catalysts are rather different in the size of Pd particles reflecting the Pd dispersion, as shown in
Figure 1,
Table 1 and
Figure S1 of the
Supplementary Materials. That is, the precursor used to carry Pd has a significant influence on the Pd particle size of resultant catalysts; for the Pd/SiO
2 catalysts calcined at 400 °C and reduced at 400 °C, palladium acetate (Pd(OAc)
2) and in particular acetylacetonate (Pd(Acac)
2) give much smaller Pd particles (7.7 and 4.6 nm, respectively), whereas palladium chloride (PdCl
2) and nitrate (Pd(NO
3)
2) lead to much larger Pd particles (12.3 and 14.5 nm, respectively).
Moreover, as shown in
Figure 1 and
Figure S1, for the Pd/SiO
2-Acac and Pd/SiO
2-OAc catalysts, the calcination treatment at 400–800 °C as well as reduction at 400 °C does not induce any marked agglomeration of Pd species and the increase in Pd particle size upon these calcination and reduction treatments is also rather insignificant. In contrast, a marked increase in the Pd particle size was observed on the Pd/SiO
2-Cl and Pd/SiO
2-NO
3 catalysts upon calcination at elevated temperature. The current results illustrate that the Pd particle size or Pd dispersion of the Pd/SiO
2 catalyst prepared by the dry ball-milling method is closely related to the Pd precursor; with palladium acetylacetonate as the precursor, the Pd/SiO
2-Acac catalyst displays a high dispersion of Pd species as well as a superior resistance against sintering upon calcination and reduction at a relatively high temperature.
The evolution of the mixture of SiO
2 support and different Pd precursors from the dry ball-milling upon later calcination at elevated temperatures were explored by in situ XRD, as demonstrated in
Figure 2. After the ball-milling, no diffraction lines are observed for Pd(Acac)
2/SiO
2 and Pd(OAc)
2/SiO
2 at 30 °C, suggesting that the Pd(Acac)
2 and Pd(OAc)
2 precursors are well dispersed on the SiO
2 support. Pd(Acac)
2 and Pd(OAc)
2 on SiO
2 start to decompose at a temperature above 150 °C, as evidenced by the appearance of diffraction lines for metallic Pd in the XRD patterns of Pd(Acac)
2/SiO
2 and Pd(OAc)
2/SiO
2 calcined at 150 °C. When the calcination temperature is elevated to above 300 °C, the diffraction peaks attributed to PdO begin to emerge and the diffraction intensity of PdO increases gradually with the increase in calcination temperature, indicating the agglomeration of PdO on Pd/SiO
2-Acac and Pd/SiO
2-OAc. As the Pd species are well dispersed on Pd(Acac)
2/SiO
2, the diffraction lines of either Pd or PdO on the low-temperature calcined Pd(Acac)
2/SiO
2 catalyst are rather weak.
In contrast, the diffraction lines for PdCl2 and PdO are detected for PdCl2/SiO2 and Pd(NO3)2/SiO2, respectively, even at 30 °C, implying that it is somewhat difficult to obtain highly dispersed PdCl2 and Pd(NO3)2 just through the mechanical ball-milling. The diffraction line of PdCl2 is replaced by the diffraction peaks of PdO phase for the PdCl2/SiO2 catalyst calcined above 400 °C. After that, for all the Pd/SiO2-R catalysts, the intensity of diffraction peaks attributed to PdO increases with increasing the calcination temperature due to the agglomeration of PdO, although four Pd/SiO2 catalysts are rather different in the agglomeration degree, consistent with the TEM results.
It is especially noteworthy that among four Pd precursors used in this work, Pd(Acac)
2 always gives the Pd/SiO
2-Acac catalyst the highest dispersion of Pd species and superior resistance against sintering upon calcination and reduction at high temperature. As reported by Van Veen and co-workers, for the thermal decomposition of supported metal acetylacetonate complexes (M(Acac)
n) on the SiO
2 support, the transfer of H from surface –OH groups to adsorbed M(Acac)
n to form Acac–H was consistently observed [
25]. As shown in
Figure S2 of the
Supplementary Materials, an absorption peak at 960 cm
−1 assigned to the Si–OH asymmetric stretching vibrations is observed in the FT-IR spectrum of the SiO
2 support [
26]; this characteristic peak of Si–OH becomes much weaker in the spectrum of Pd/SiO-Acac loaded with the Pd(acac)
2 precursor, indicating the formation of Acac-H. At the same time, the palladium species was reduced to highly dispersed metallic Pd (with very weak diffraction signals in
Figure 2) by the Acac ligand at elevated temperature (about 200 °C). In addition, the TG-MS curves shown in
Figure S3 of the
Supplementary Materials also illustrate that there is a steep weight loss at 198 °C for Pd(Acac)
2/SiO
2, accompanied with a sharp DTG peak as well as sharp mass spectroscopy signals for CO
2 and H
2O, indicating the thermal decomposition of Acac ligands supported on SiO
2, consistent with the in situ XRD results (
Figure 2).
2.2. Performance of the Pd/SiO2 Catalysts Prepared by the Dry Ball-Milling Method in Lean Methane Oxidation
The overall performance of various Pd/SiO
2-
R catalysts prepared by the dry ball-milling method with different Pd precursors in the lean methane oxidation was compared by the light-off tests, as shown in
Figure 3A and
Figure S4 of the
Supplementary Materials. Apparently, four Pd/SiO
2 catalysts prepared with different Pd precursors are also rather different in the catalytic activity for methane oxidation. The Pd/SiO
2-Acac catalyst prepared with palladium acetylacetonate performs the best in lean methane oxidation; the temperatures corresponding to a methane conversion of 10% (
T10%), 50% (
T50%) and 90% (
T90%) are 242, 272 and 288 °C, respectively, and a complete methane conversion (
T100%) is achieved at about 310 °C. Next is the Pd/SiO
2-OAc catalyst prepared from palladium acetate, with
T10%,
T50%,
T90% and
T100% values of 245, 280, 305 and 335 °C, respectively. The third is the Pd/SiO
2-Cl catalyst prepared from palladium chloride, with a
T90% value of 345 °C. The Pd/SiO
2-NO
3 catalyst prepared from palladium nitrate performs poorest, with a
T90% value of 464 °C. That is, the catalytic activity of various Pd/SiO
2 catalysts follows the order of Pd/SiO
2-Acac > Pd/SiO
2-OAc > Pd/SiO
2-Cl >> Pd/SiO
2-NO
3; such an order of catalytic activity in methane oxidation is consistent with that of Pd dispersion or Pd particle size [
9]; that is, the catalytic activity increases with an increase in the Pd dispersion or a decrease in the Pd particle size.
Interestingly, as shown in
Figure S4, four Pd/SiO
2-
R catalysts prepared from different Pd precursors are also rather different in the thermal stability against calcination treatment at elevated temperatures. For the Pd/SiO
2-Acac catalyst, it seems that the calcination at a temperature below 800 °C has little impact on its catalytic activity in methane oxidation, according well with its superior resistance against sintering upon calcination at elevated temperatures. In contrast, the Pd/SiO
2-OAc catalyst displays a moderate decrease in the activity for methane oxidation upon calcination at a temperature higher than 600 °C, whereas such a calcination treatment has a significant detrimental influence on the catalytic activity of Pd/SiO
2-Cl and Pd/SiO
2-NO
3 in lean methane oxidation, in accordance with the serious agglomeration of Pd species on Pd/SiO
2-Cl and Pd/SiO
2-NO
3 at elevated temperatures, as demonstrated by the XRD and TEM results (
Figure 2 and
Figure S1). Besides the catalytic activity, the thermal stability of Pd/SiO
2 catalysts also follows the order of Pd/SiO
2-Acac > Pd/SiO
2-OAc >> Pd/SiO
2-Cl > Pd/SiO
2-NO
3, indicating that palladium acetylacetonate is an appropriate precursor, much superior to palladium acetate, chloride and nitrate, to prepare the Pd/SiO
2 catalyst with high activity and stability for lean methane oxidation through the dry ball-milling method.
In addition, a rough comparison of current Pd/SiO
2-Acac catalyst with some other supported palladium catalysts in their activity for lean methane oxidation was given in
Table S1 of the Supplementary Materials. Obviously, current Pd/SiO
2-Acac catalyst prepared by the simple dry ball-milling method exhibits rather high activity in the lean methane oxidation, compared with most of those catalysts reported in the open literature.
2.3. Effect of Pd Chemical State on the Catalytic Activity of Pd/SiO2 in Lean Methane Oxidation
The activity of Pd/SiO
2-Acac catalyst in reduced state and the PdO/SiO
2-Acac catalyst in oxidized state in lean methane oxidation is compared in
Figure 3B. Obviously, the oxidation state of Pd species does have a certain influence on the catalytic activity of PdO/SiO
2. The PdO/SiO
2-Acac catalyst in oxidized state (calcined at 400 °C, with a
T90% value of 313 °C) exhibits a lower activity than the Pd/SiO
2-Acac catalyst in reduced state (reduced at 400 °C, with a
T90% value of 287 °C). It suggests that Pd
0 on the supported Pd/SiO
2 catalyst is more active than PdO for the lean methane oxidation. However, it is noteworthy that during the light-off tests for methane oxidation, oxygen is always highly excessive and the reduced Pd
0 species could be oxidized to PdO in the oxygen-rich ambiance (the oxygen concentration in the reaction stream is in general higher than 18 vol.%) at elevated temperatures. This is proved by the in situ XRD patterns of the Pd/SiO
2-Acac catalysts that come through the lean methane oxidation reaction at different temperatures, as shown in
Figure S5A of the
Supplementary Materials. It means that the actual working Pd species in lean methane oxidation may include the PdO
x species even when the reduced Pd/SiO
2 catalyst with only Pd
0 species is initially used. Moreover, it seems that the content of PdO increases with the increase in reaction temperature and the PdO species rather than Pd
0 turns out to be the primary Pd species for lean methane oxidation at a temperature above 320 °C.
To evaluate the effect of the Pd chemical state on the catalytic activity of Pd/SiO
2 in lean methane oxidation, various Pd/SiO
2-Acac catalysts with different Pd oxidation states were tested at a fixed low temperature of 230 °C. As demonstrated in
Figure S5A, a temperature below 240 °C for the lean methane oxidation does not induce a serious transformation of the Pd
0 species to PdO in a certain reaction period (about several hours).
A series of PdO
x/SiO
2-Acac catalysts was first prepared by oxidizing the previously reduced Pd/SiO
2-Acac in air for 30 min at different temperatures (240–500 °C), which was then characterized by Pd 3d XPS to determine the value of
x = [PdO]/([Pd] + [PdO]), as illustrated in
Table 2 and
Figure S6 of the
Supplementary Materials. The TEM images shown in
Figure S7 of the
Supplementary Materials illustrate that such an oxidation treatment at 240–500 °C has little impact on the catalyst morphology and Pd dispersion; all the PdO
x/SiO
2-Acac catalysts in this series have a similar Pd particle size, in the range of 4.2–4.8 nm, as given in
Table 2.
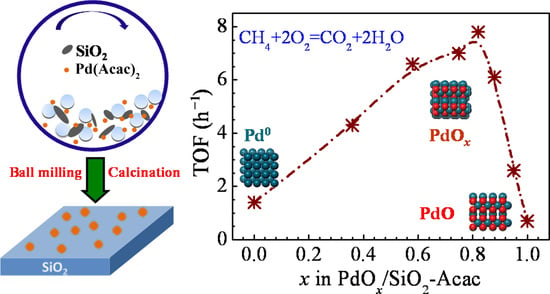
When this series of PdO
x/SiO
2-Acac catalysts is employed in the lean methane oxidation, as shown in
Figure 4A,C, the catalytic activity is closely connected with the oxidation state of Pd species (represented by the value of
x). It seems that PdO/SiO
2-Acac (
x = 1) has the lowest activity (with a turnover frequency (TOF) of 0.7 h
−1) and Pd/SiO
2-Acac (
x = 0) shows slightly higher activity (with a TOF of 1.4 h
−1) than PdO/SiO
2-Acac. In contrast, the PdO
x/SiO
2-Acac catalysts (
x = 0.36–0.95) exhibit much higher activity for the lean methane oxidation at low temperature (230 °C). That is, the activity of various Pd species follows the order of PdO
x >> Pd > PdO. In particular, the PdO
0.82/SiO
2-Acac catalyst is most active, with the highest TOF (7.8 h
−1) and methane conversion (5.5%).
Next, to help reveal the nature of the PdO
x species on Pd/SiO
2, another series of
xPdO
+ (1 −
x)Pd/SiO
2-Acac catalysts (
x = 0–1) was prepared by mixing the reduced (1 −
x)Pd/SiO
2-Acac portion and oxidized
xPdO/SiO
2-Acac portion through grinding; both the Pd and PdO phases could be detected in their XRD patterns, as shown in
Figure S5B of the Supplementary Materials. When this series of
xPdO
+ (1 −
x)Pd/SiO
2-Acac catalysts is tested in lean methane oxidation, as shown in
Figure 4B,C, the
xPdO
+ (1 −
x)Pd/SiO
2-Acac catalysts (
x = 0.2, 0.4, 0.6 and 0.8) show similar activity to PdO/SiO
2-Acac and Pd/SiO
2-Acac (with the TOF of 1–2 h
−1); in most cases, slightly more active than Pd/SiO
2-Acac and less active than PdO/SiO
2-Acac. It indicates that a simple mixing of the reduced Pd/SiO
2-Acac and oxidized PdO/SiO
2-Acac catalysts does not give any synergy between the Pd
0 and PdO species in two catalysts for the lean methane oxidation. The high catalytic activity of PdO
x/SiO
2-Acac should be related to its particular structure and properties with a tight interaction between the Pd atoms of different valence states.
The H
2-TPR profiles of PdO
x/SiO
2-Acac with different
x values are presented in
Figure 5A. Two intense peaks are observed; a hydrogen consumption peak at −10–40 °C probably involves a superposition of the reduction of PdO species to the metal Pd
0 species and the formation of palladium hydride (PdH
x) from the Pd
0 species, whereas another is a hydrogen desorption peak at 45–75 °C, corresponding to the decomposition of palladium hydride [
27]. As shown in
Figure 5A, the PdO/SiO
2-Acac catalyst displays the highest reduction temperature compared to the PdO
x/SiO
2-Acac catalysts. In particular, the PdO
0.82/SiO
2-Acac catalyst shows the lowest reduction temperature, indicating a superior reducibility, which allows a quick redox cycle and then gives it a high activity for the lean methane oxidation at low temperature.
A long-term catalytic test was then performed for the lean methane oxidation over the PdO
0.82/SiO
2-Acac catalyst at 280 °C, as shown in
Figure 5B. Obviously, the catalytic activity of PdO
0.82/SiO
2-Acac is degraded gradually with the time on stream; the fresh PdO
0.82/SiO
2-Acac catalyst shows a methane conversion of 49.6%, which decreases to 28.1% after reaction at 280 °C for 60 h. However, when the degraded PdO
0.82/SiO
2-Acac catalyst is re-treated according to the procedures for preparing the fresh PdO
0.82/SiO
2-Acac catalyst (viz., reduced by H
2 at 400 °C for 1 h and re-oxidized by air at 350 °C for 0.5 h), the activity of the degraded PdO
0.82/SiO
2-Acac catalyst is completely recovered and the conversion of methane comes back to 50.2%.
For the supported palladium catalysts in lean methane oxidation, the degradation of catalytic activity may stem from two main causes: state transformation of active Pd species and the sintering of Pd particles. In comparison with the fresh PdO
0.82/SiO
2-Acac catalyst, after enduring the lean methane oxidation at 280 °C for 60 h, as shown in
Figure S8 of the
Supplementary Materials, no significant change in the catalyst morphology and Pd particle size is observed on the spent PdO
0.82/SiO
2-Acac catalyst by the TEM images, whereas the chemical state of Pd changes from PdO
0.82/SiO
2 to PdO/SiO
2, as evidenced by the Pd 3d XPS spectra. These results indicate that the degradation of PdO
0.82/SiO
2-Acac in catalytic activity during the lean methane oxidation at low temperature is primarily ascribed to the over oxidation of PdO
x species, rather than any change in the Pd particle size; nevertheless, such a degradation process is reversible and the reactivity of the degraded PdO
0.82/SiO
2-Acac catalyst can be completely recovered when the oxidation state of Pd species is resumed to original PdO
0.82 through proper redox treatments.
2.4. More about the Active PdOx Species in Lean Methane Oxidation
The active phase of Pd in lean methane oxidation is a longstanding puzzle. Some recent studies suggest that Pd
0 is the “most active phase” in lean methane oxidation [
9,
15]. However, the state of metallic Pd changes gradually along with the reaction course in the oxygen-rich ambiance, and the PdO
x or even PdO species was probably the actual working Pd species in the lean methane oxidation at elevated temperatures [
28,
29]. Hellman and co-workers observed that the efficient dissociation of methane required sufficiently thick PdO(101) films or metallic Pd [
11], consistent with the result of DFT calculation that either under-coordinated Pd sites in PdO and metallic Pd surfaces displayed low activation energies for methane oxidation. This phenomenon was also observed for CO oxidation; multilayer PdO(101) was intrinsically much more active in CO oxidation than the single-layer PdO(101)/Pd(100) [
30]. For the lean methane oxidation at a rather low temperature (<300 °C), which is mainly concerned in this work, when the metallic Pd species is exposed to the oxygen-rich atmosphere, a monolayer of Pd oxide may be generated immediately. Along with the reaction course, a Pd oxide shell around the metal core is formed and its thicknesses increase with the increase in reaction temperature and time on stream, and eventually, bulk PdO may be formed [
4].
DFT calculation was further performed to elucidate the intrinsic relationship between the reactivity of methane oxidation and the active surface Pd species, as illustrated in
Figure S9 of the
Supplementary Materials. As the methane molecule is highly stable and has a weak interaction with the catalyst surfaces, the dissociative adsorption of methane is assumed as the rate determining step in the catalytic cycle of methane oxidation. The most stable bulk Pd(100), PdO(100) and three monolayer PdO(101)/Pd(100) (3ML, labeled as PdO
0.75, where the atomic ratio of O to Pd is 0.75) are modeled to mimic the surface Pd
0, PdO and PdO
x, respectively, as PdO(101) is the stable surface for an epitaxially grown PdO film on Pd(100) [
12,
31,
32,
33].
The structures of reactant, transition state and product as well as energy change for the dissociative adsorption of methane on the Pd(100), PdO(100) and PdO
0.75 (3ML-PdO(101)/Pd(100)) surfaces are comparatively presented in
Table S2 of the
Supplementary Materials. On Pd(100), methane is weakly absorbed; a four-center transition state (H
3C–Pd–H–Pd) is formed upon electron transfer from the carbon–hydrogen bond to the Pd atom, which is further decomposed into Pd–CH
3 and Pd–H. On both 3ML-PdO(101)/Pd(100) and PdO(100), the exposed Pd atom is inserted into a C–H bond of CH
4 and the H atom is concurrently abstracted by the vicinal O species, forming the four-center transition state (H
3C–Pd–H–O), which is then decomposed into Pd–CH
3 and OH. Apparently, the PdO
0.75 surface exhibits the lowest energy barrier (0.73 eV) and reaction energy (−0.33 eV) for the methane dissociation; in contrast, the PdO(100) surface displays the highest energy barrier (1.27 eV). Meanwhile, although Pd(100) shows a similar energy barrier to PdO
0.75, the reaction over the former Pd(100) surface is endothermic by 0.53 eV, thermodynamically unfavorable. In addition, Pd(100) is usually shown as O*–Pd(100) with high O coverage, because the adsorption energy of O
2 on Pd(100) is up to −2.0 eV and the energy barrier of O
2 dissociation is only 0.03 eV [
31]; nevertheless, the energy barrier for methane dissociation on O*–Pd (100) is 1.0–1.5 eV, still much higher than that on PdO
0.75 [
7]. That is, the DFT calculation results also prove that the PdO
x species is much more active than Pd
0 and PdO in the dissociation of methane.
Notably, Sui and co-workers observed a non-monotonic pressure dependence of the catalytic reaction rate in the range of 0.1–1.2 MPa [
34], a behavior contrast to other noble metals (Pt and Rh) where the methane reaction rates always increased with rising pressure. The reactions were conducted at 627 °C and the volume contents of methane and oxygen were 4.9–6.0% and 24.8–40.7%, respectively. Similarly, through first-principle microkinetics modeling, Floren and co-workers observed different pressure dependences of intrinsic TOF at three temperature regimes [
35]. At a temperature below 420 °C or above 475 °C, a negative or positive pressure dependency (in the range of 0.1–1.0 MPa) of intrinsic TOF was observed, respectively. In the intermediate temperature regime (420–475 °C), the effect of total pressure on the TOF showed a temperature- and pressure-dependent maximum. Meanwhile, when the impact of mass and heat transport on the TOF was taken into account, the intermediate temperature regime spanned a narrower range of 400–430 °C. Such a non-monotonic pressure dependence behavior was attributed to the pressure-dependent coverages of the dominant surface species such as water, bicarbonates and hydroxyls.
In this work, a series of PdO
x/SiO
2-Acac catalysts with similar Pd particle sizes but different oxidation states was prepared through proper redox treatments, to mimic various Pd species of different valence states in a working catalyst in lean methane oxidation. Their catalytic activity was evaluated at a rather low temperature of 230 °C and atmospheric pressure, to allow no significant change in the Pd state in a short period. Meanwhile, a low methane feed concentration (1 vol.%, dry feed, without co-feeding any H
2O and CO
2) and a low methane conversion (<6%) allow one to have a right evaluation on the intrinsic catalytic activity of various PdO
x species. In this case, the coverage of surface water, bicarbonates and hydroxyls species as well as the impact of mass and heat transport should be rather minor. The results accord well with previous reports that Pd
0 is more active than PdO. Moreover, the catalytic activity is closely related to the oxidation state of PdO
x species (representing by the value of
x) and the activity of various Pd species follows the order of PdO
x >> Pd > PdO. The PdO
x/SiO
2-Acac catalysts (in particular for PdO
0.82/SiO
2-Acac when
x = 0.82) exhibit much higher activity for lean methane oxidation at low temperature than Pd/SiO
2-Acac and PdO/SiO
2-Acac. As a result, we believe that it is just a coincidence between the non-monotonic dependence of the catalytic activity on the Pd oxidation state observed in this work and the non-monotonic pressure dependence of the catalytic reaction rate reported by Sui, Floren and co-workers [
34,
35]. In their cases, it seems that PdO was always the dominant active species during the whole reaction courses, whereas in this work, PdO
x/SiO
2 with different Pd oxidation states was used and the oxidation state of Pd may even change during the reaction course. Two phenomena should be rather different in their intrinsic causation, though both reveal the mechanical complexity of methane oxidation over the supported Pd catalysts.
In addition, for the lean methane oxidation at low temperature, the catalytic activity of PdOx/SiO2-Acac is degraded considerably along with the reaction, owing primarily to the over oxidation of PdOx species. However, such a degradation process is reversible and the reactivity of the spent PdOx/SiO2-Acac catalyst can be completely recovered when the oxidation state of PdO species is resumed to original PdOx through proper redox treatments.