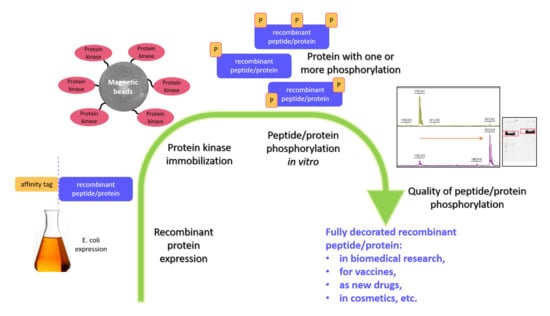
Contemporary Enzyme-Based Methods for Recombinant Proteins In Vitro Phosphorylation
Abstract
:1. Introduction
2. In Vitro Phosphorylation of Recombinant Proteins: Various Methodological Approaches
- Fmoc SPSS using modified peptides
- SPSS combined with microwave radiation
- Protein semisynthesis
- Protein synthesis and biotechnology combination
- Enzyme-based phosphorylation approach
3. Overview of Protein Kinases and Phosphatases Suitable for In Vitro Phosphorylation
4. In Vitro Protein Phosphorylation by Immobilized Kinases
5. Monitoring Kinase Activity and Quality of Peptide/Protein Phosphorylation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AGC | PKA, PKG and PKC family protein group |
| ALP | alkaline phosphatase |
| BLK | B lymphoid tyrosine kinase |
| CAMK | the Ca2+/calmodulin-dependent protein kinase group |
| CDK | cyclin dependent kinase |
| CFPS | cell-free protein synthesis |
| Chk1, Chk2 | checkpoint kinases 1 and 2 |
| CK1 | casein kinase 1 group |
| CKIIα | α-casein kinase II |
| CLK | Cdc2-like kinase family |
| CMGC | group of MAPK, CDK, GSK3, CLK |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular-regulated kinase |
| ESI MS | electrospray ionization mass spectrometry |
| FAK | focal adhesion kinase |
| GSK-3 | glycogen synthase kinase-3 |
| GST | glutathione S-Transferase |
| HPLC | high performance liquid chromatography |
| ITK | interleukin 2-inducible T cell kinase |
| JNK | c-Jun amino N-terminal kinase |
| LCK | lymphocyte specific protein tyrosine kinase |
| MAPK | mitogen-activated protein kinase |
| MEK, MKK | mitogen-activated protein kinases kinase |
| MS | mass spectrometry |
| NFH-SA | neurofilament heavy subunit |
| PMA | phorbol myristate acetate |
| PP | protein phosphatase |
| PKA, PKC, PKG, PKR | protein kinase A, C, G, and R |
| PTM | posttranslational modification |
| SPR | surface plasmon resonance |
| SPSS | solid-phase peptide synthesis |
| STE | yeast kinase homologues Sterile 7, Sterile 11, Sterile 20 |
| STK | serine/threonine kinases |
| TK | tyrosine kinases |
| TKL | tyrosine kinases like |
| TPK3 | toxoplasma protein kinase-3 |
| TTP | tristetraprolin |
References
- Walsh, G. Posttranslational Modifications to Improve Biopharmaceuticals. Mod. Biopharm. 2013, 445–467. [Google Scholar] [CrossRef]
- Qiu, Y.; Poppleton, E.; Mekkat, A.; Yu, H.; Banerjee, S.; Wiley, S.E.; Dixon, J.E.; Kaplan, D.L.; Lin, Y.-S.; Brodsky, B. Enzymatic Phosphorylation of Ser in a Type I Collagen Peptide. Biophys. J. 2018, 115, 2327–2335. [Google Scholar] [CrossRef] [Green Version]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, W.X. Proteomics approaches to understand protein phosphorylation in pathway modulation. Curr. Opin. Plant. Biol. 2010, 13, 279–286. [Google Scholar] [CrossRef]
- Zakhartchouk, A.N.; Viswanathan, S.; Mahony, J.B.; Gauldie, J.; Babiuk, L.A. Severe acute respiratory syndrome coronavirus nucleocapsid protein expressed by an adenovirus vector is phosphorylated and immunogenic in mice. J. Gen. Virol. 2005, 86, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pierobon, M.; Petricoin, E.F., III; Wulfkuhle, J.D. Phosphoprotein-based drug target activation mapping for precision oncology: A view to the future. Expert Rev. Proteom. 2018, 15, 851–853. [Google Scholar] [CrossRef] [Green Version]
- Fields, G.B. Introduction to Peptide Synthesis. Curr. Protoc. Protein Sci. 2001, 26, 58–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, G. Post-translational modifications of protein biopharmaceuticals. Drug Discov. Today 2010, 15, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Rodney, J.Y.H.; Milo, G. Transforming proteins and genes into drugs. In Biotechnology and Biopharmaceuticals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; p. 1. ISBN 9781118660485. [Google Scholar]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overton, T.W. Recombinant protein production in bacterial hosts. Drug Discov. Today 2014, 19, 590–601. [Google Scholar] [CrossRef]
- Clark, D.P.; Pazdernik, N.J. Chapter 10—Recombinant Proteins. In Biotechnology, 2nd ed.; Academic Cell: Boston, MA, USA, 2016; pp. 335–363. ISBN 978-0-12-385015-7. [Google Scholar]
- Abd-Aziz, N.; Tan, B.C.; Rejab, N.A.; Othman, R.Y.; Khalid, N. A New Plant Expression System for Producing Pharmaceutical Proteins. Mol. Biotechnol. 2020, 62, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, H.; Mazur, X.; Fussenegger, M.; Bailey, J.E. Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotechnol. Bioeng. 1999, 63, 573–582. [Google Scholar] [CrossRef]
- Go, E.P.; Liao, H.-X.; Alam, S.M.; Hua, D.; Haynes, B.F.; Desaire, H. Characterization of Host-Cell Line Specific Glycosylation Profiles of Early Transmitted/Founder HIV-1 gp120 Envelope Proteins. J. Proteome Res. 2013, 12, 1223–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freivalds, J.; Dislers, A.; Ose, V.; Pumpens, P.; Tars, K.; Kazaks, A. Highly efficient production of phosphorylated hepatitis B core particles in yeast Pichia pastoris. Protein Expr. Purif. 2011, 75, 218–224. [Google Scholar] [CrossRef]
- Yoshida, H.; Hastie, J.; Mclauchlan, H.; Cohen, P.; Goedert, M. Phosphorylation of microtubule-associated protein tau by isoforms of c-Jun N-terminal kinase (JNK). J. Neurochem. 2004, 90, 352–358. [Google Scholar] [CrossRef]
- Cao, H.; Lin, R. Phosphorylation of Recombinant Tristetraprolin In vitro. Protein J. 2008, 27, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Palomares, L.A.; Estrada-Moncada, S.; Ramírez, O.T. Production of Recombinant Proteins BT. In Recombinant Gene Expression: Reviews and Protocols; Balbás, P., Lorence, A., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 15–51. ISBN 978-1-59259-774-1. [Google Scholar]
- Raghow, R.; Dong, Q.; Elam, M.B. Phosphorylation dependent proteostasis of sterol regulatory element binding proteins. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2019, 1864, 1145–1156. [Google Scholar] [CrossRef]
- Cohen, P. The role of protein phosphorylation in human health and disease: Delivered on June 30th 2001 at the FEBS Meeting in Lisbon. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L.L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Haycock, J.W.; Ahn, N.G.; Cobb, M.H.; Krebs, E.G. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc. Natl. Acad. Sci. USA 1992, 89, 2365–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeranna; Amin, N.D.; Ahn, N.G.; Jaffe, H.; Winters, C.A.; Grant, P.; Pant, H.C. Mitogen-Activated Protein Kinases (Erk1,2) Phosphorylate Lys-Ser-Pro (KSP) Repeats in Neurofilament Proteins NF-H and NF-M. J. Neurosci. 1998, 18, 4008–4021. [Google Scholar] [CrossRef]
- Ferreira, A.; López, E.; Matthiesen, R.; López, I.; Ashman, K.; Mendieta, J.; Wesselink, J.-J.; Gómez-Puertas, P. Functional phosphoproteomics for current immunology research. J. Integr. OMICS 2011, 1, 1–16. [Google Scholar] [CrossRef]
- Drewes, G.; Lichtenberg-Kraag, B.; Döring, F.; Mandelkow, E.M.; Biernat, J.; Goris, J.; Dorée, M.; Mandelkow, E. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992, 11, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Wang, X.; Siedlak, S.L.; Perry, G.; Smith, M.A.; Zhu, X. c-Jun phosphorylation in Alzheimer disease. J. Neurosci. Res. 2007, 85, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Ngoei, K.R.W.; Catimel, B.; Milech, N.; Watt, P.M.; Bogoyevitch, M.A. A novel retro-inverso peptide is a preferential JNK substrate-competitive inhibitor. Int. J. Biochem. Cell Biol. 2013, 45, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Shchemelinin, I.; Šefc, L.; Nečas, E. Protein kinases, their function and implication in cancer and other diseases (Review). Folia Biol. 2006, 52, 81–101. [Google Scholar]
- Cohen, P. Immune diseases caused by mutations in kinases and components of the ubiquitin system. Nat. Immunol. 2014, 15, 521–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, R.J.; Stylli, S.S.; Luwor, R.B.; Kaye, A.H.; Hovens, C.M. Glycogen synthase kinase-3β (GSK-3β) and its dysregulation in glioblastoma multiforme. J. Clin. Neurosci. 2013, 20, 1185–1192. [Google Scholar] [CrossRef]
- Stoothoff, W.H.; Johnson, G.V.W. Tau phosphorylation: Physiological and pathological consequences. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2005, 1739, 280–297. [Google Scholar] [CrossRef] [Green Version]
- Stachyra, A.; Grzelak, S.; Basałaj, K.; Zawistowska-Deniziak, A.; Bień-Kalinowska, J. Immunization with a Recombinant Protein of Trichinella britovi 14-3-3 Triggers an Immune Response but No Protection in Mice. Vaccines 2020, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Norais, N.; Hall, J.A.; Gross, L.; Tang, D.; Kaur, S.; Chamberlain, S.H.; Burke, R.L.; Marcus, F. Evidence for a phosphorylation site in cytomegalovirus glycoprotein gB. J. Virol. 1996, 70, 5716–5719. [Google Scholar] [CrossRef] [Green Version]
- Fauvel, B.; Yasri, A. Antibodies directed against receptor tyrosine kinases: Current and future strategies to fight cancer. mAbs 2014, 6, 838–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smet-Nocca, C.; Wieruszeski, J.-M.; Buée, L.; Landrieu, I.; Lippens, G. Regulation of Pin1 peptidyl-prolylcis/transisomerase activity by its WW binding module on a multi-phosphorylated peptide of Tau protein. FEBS Lett. 2005, 579, 4159–4164. [Google Scholar] [CrossRef] [Green Version]
- Højlys-Larsen, K.B.; Jensen, K.J. Solid-Phase Synthesis of Phosphopeptides. Methods Mol. Biol. 2013, 1047, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mora, N.; Lacombe, J.M.; Pavia, A.A. A new approach to phosphoserine and phosphothreonine synthons suitable for the stepwise synthesis of phosphopeptides. Tetrahedron Lett. 1993, 34, 2461–2464. [Google Scholar] [CrossRef]
- Samarasimhareddy, M.; Mayer, D.; Metanis, N.; Veprintsev, D.; Hurevich, M.; Friedler, A. A targeted approach for the synthesis of multi-phosphorylated peptides: A tool for studying the role of phosphorylation patterns in proteins. Org. Biomol. Chem. 2019, 17, 9284–9290. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.W.R.; Williams, G.M.; Shepherd, P.; Brimble, M.A. The Synthesis of Phosphopeptides Using Microwave-assisted Solid Phase Peptide Synthesis. Int. J. Pept. Res. Ther. 2008, 14, 387–392. [Google Scholar] [CrossRef]
- Thompson, R.E.; Muir, T.W. Chemoenzymatic Semisynthesis of Proteins. Chem. Rev. 2020, 120, 3051–3126. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.D.; Gan, R.; Hodgman, C.E.; Jewett, M.C. Cell-free protein synthesis: Applications come of age. Biotechnol. Adv. 2012, 30, 1185–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oza, J.P.; Aerni, H.R.; Pirman, N.L.; Barber, K.W.; Ter Haar, C.M.; Rogulina, S.; Amrofell, M.B.; Isaacs, F.J.; Rinehart, J.; Jewett, M.C. Robust production of recombinant phosphoproteins using cell-free protein synthesis. Nat. Commun. 2015, 6, 8168. [Google Scholar] [CrossRef] [Green Version]
- Lei, R.; Lee, J.P.; Francis, M.B.; Kumar, S. Structural Regulation of a Neurofilament-Inspired Intrinsically Disordered Protein Brush by Multisite Phosphorylation. Biochemistry 2018, 57, 4019–4028. [Google Scholar] [CrossRef]
- Goux, M.; Fateh, A.; Defontaine, A.; Cinier, M.; Tellier, C. In vivo phosphorylation of a peptide tag for protein purification. Biotechnol. Lett. 2016, 38, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Lei, R. Multi-site Phosphorylation Modulates the Conformation and Electrostatic Response of Intrinsically Disordered Protein Brushes. Biophys. J. 2018, 114, 590a. [Google Scholar] [CrossRef]
- Srinivasan, N.; Bhagawati, M.; Ananthanarayanan, B.; Kumar, S. Stimuli-sensitive intrinsically disordered protein brushes. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, G.; Takeuchi, H.; Nagano, K.; Gao, J.; Ohyama, Y.; Mori, Y.; Hirata, M. Regulated Interaction of Protein Phosphatase 1 and Protein Phosphatase 2A with Phospholipase C-Related but Catalytically Inactive Protein. Biochemistry 2012, 51, 3394–3403. [Google Scholar] [CrossRef]
- Liu, H.; Queffélec, C.; Charlier, C.; Defontaine, A.; Fateh, A.; Tellier, C.; Talham, D.R.; Bujoli, B. Design and Optimization of a Phosphopeptide Anchor for Specific Immobilization of a Capture Protein on Zirconium Phosphonate Modified Supports. Langmuir 2014, 30, 13949–13955. [Google Scholar] [CrossRef]
- Lan, Y.T.; Li, J.; Liao, W.-Y.; Ou, J.-H. Roles of the Three Major Phosphorylation Sites of Hepatitis B Virus Core Protein in Viral Replication. Virology 1999, 259, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Roux, P.P.; Thibault, P. The Coming of Age of Phosphoproteomics—from Large Data Sets to Inference of Protein Functions. Mol. Cell. Proteom. 2013, 12, 3453–3464. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Alicea-Velázquez, N.L.; Bannwarth, L.; Lehtonen, S.I.; Boggon, T.J.; Cheng, H.-C.; Hytönen, V.P.; Turk, B.E. Global Analysis of Human Nonreceptor Tyrosine Kinase Specificity Using High-Density Peptide Microarrays. J. Proteome Res. 2014, 13, 4339–4346. [Google Scholar] [CrossRef] [Green Version]
- Kobe, B.; Kampmann, T.; Forwood, J.K.; Listwan, P.; Brinkworth, R.I. Substrate specificity of protein kinases and computational prediction of substrates. Biochim. Biophys. Acta BBA Proteins Proteom. 2005, 1754, 200–209. [Google Scholar] [CrossRef]
- Ubersax, J.A.; Ferrell, J.E., Jr. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V.; Kornhauser, J.M.; Latham, V.; Murray, B.; Nandhikonda, V.; Nord, A.; Skrzypek, E.; Wheeler, T.; Zhang, B.; Gnad, F. 15 years of PhosphoSitePlus®: Integrating post-translationally modified sites, disease variants and isoforms. Nucleic Acids Res. 2019, 47, D433–D441. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.A.; Hunter, T. Kinomics: Methods for deciphering the kinome. Nat. Methods 2005, 2, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, S.; Borchers, C.H. Protein species-specific characterization of conformational change induced by multisite phosphorylation. J. Proteom. 2016, 134, 138–143. [Google Scholar] [CrossRef]
- Lee, J.; Samson, A.A.S.; Song, J.M. Direct On-Paper Inkjet Printing of Kinase-to-Kinase Phosphorylation Cascade Reactions. ACS Omega 2019, 4, 7866–7873. [Google Scholar] [CrossRef]
- Hromadkova, L.; Kupcik, R.; Vajrychova, M.; Prikryl, P.; Charvatova, A.; Jankovicova, B.; Ripova, D.; Bilkova, Z.; Slovakova, M. Kinase-loaded magnetic beads for sequential in vitro phosphorylation of peptides and proteins. Analyst 2018, 143, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Chen, Y.; Mei, Z.; Xiao, Y. Screening of inhibitors of glycogen synthase kinase-3β from traditional Chinese medicines using enzyme-immobilized magnetic beads combined with high-performance liquid chromatography. J. Chromatogr. A 2015, 1425, 8–16. [Google Scholar] [CrossRef]
- Reddy, C.E.; Albanito, L.; De Marco, P.; Aiello, D.; Maggiolini, M.; Napoli, A.; Musti, A.M. Multisite phosphorylation of c-Jun at threonine 91/93/95 triggers the onset of c-Jun pro-apoptotic activity in cerebellar granule neurons. Cell Death Dis. 2013, 4, e852. [Google Scholar] [CrossRef]
- Quintero, O.A.; Unrath, W.C.; Stevens, S.M.J.; Manor, U.; Kachar, B.; Yengo, C.M. Myosin 3A Kinase Activity Is Regulated by Phosphorylation of the Kinase Domain Activation Loop. J. Biol. Chem. 2013, 288, 37126–37137. [Google Scholar] [CrossRef] [Green Version]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [Green Version]
- Zolnierowicz, S.; Bollen, M. Protein phosphorylation and protein phosphatases De Panne, Belgium, 19–24 September 1999. EMBO J. 2000, 19, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Moorhead, G.B.G.; De Wever, V.; Templeton, G.; Kerk, D. Evolution of protein phosphatases in plants and animals. Biochem. J. 2009, 417, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Terro, F. Tau protein phosphatases in Alzheimer’s disease: The leading role of PP2A. Ageing Res. Rev. 2013, 12, 39–49. [Google Scholar] [CrossRef]
- Mendoza, J.; Sekiya, M.; Taniguchi, T.; Iijima, K.M.; Wang, R.; Ando, K. Global Analysis of Phosphorylation of Tau by the Checkpoint Kinases Chk1 and Chk2in vitro. J. Proteome Res. 2013, 12, 2654–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, S.; Davis, R.J.; McLaren, A.; Cohen, P. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J. 2003, 22, 3876–3886. [Google Scholar] [CrossRef] [Green Version]
- Veeranna; Yang, D.-S.; Lee, J.-H.; Vinod, K.Y.; Stavrides, P.; Amin, N.D.; Pant, H.C.; Nixon, R.A. Declining phosphatases underlie aging-related hyperphosphorylation of neurofilaments. Neurobiol. Aging 2011, 32, 2016–2029. [Google Scholar] [CrossRef] [Green Version]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta BBA Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Dunning, C.J.; McGauran, G.; Willén, K.; Gouras, G.K.; O’Connell, D.J.; Linse, S. Direct High Affinity Interaction between Aβ42 and GSK3α Stimulates Hyperphosphorylation of Tau. A New Molecular Link in Alzheimer’s Disease? ACS Chem. Neurosci. 2016, 7, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.J.; Launer-Felty, K.; Cole, J.L. Analysis of PKR–RNA Interactions by Sedimentation Velocity. Methods Enzymol. 2011, 488, 59–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimer, L.; Betzer, C.; Kofoed, R.H.; Volbracht, C.; Fog, K.; Kurhade, C.; Nilsson, E.; Överby, A.K.; Jensen, P.H. PKR kinase directly regulates tau expression and Alzheimer’s disease-related tau phosphorylation. Brain Pathol. 2021, 31, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.A.; Thompson, M.J.; Lai, W.S.; Blackshear, P.J. Phosphorylation of Tristetraprolin, a Potential Zinc Finger Transcription Factor, by Mitogen Stimulation in Intact Cells and by Mitogen-activated Protein Kinase in Vitro. J. Biol. Chem. 1995, 270, 13341–13347. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Giorgianni, F.; Deng, X.; Beranova-Giorgianni, S.; Bridges, D.; Park, E.A.; Raghow, R.; Elam, M.B. Phosphorylation of sterol regulatory element binding protein-1a by protein kinase A (PKA) regulates transcriptional activity. Biochem. Biophys. Res. Commun. 2014, 449, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, X.; Gao, X.; Shen, Q.W.; Du, M.; Zhang, D. Phosphorylation prevents in vitro myofibrillar proteins degradation by μ-calpain. Food Chem. 2017, 218, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Benaim, G.; Villalobo, A. Phosphorylation of calmodulin. Functional implications. Eur. J. Biochem. 2002, 269, 3619–3631. [Google Scholar] [CrossRef] [PubMed]
- Cinier, M.; Petit, M.; Pecorari, F.; Talham, D.R.; Bujoli, B.; Tellier, C. Engineering of a phosphorylatable tag for specific protein binding on zirconium phosphonate based microarrays. JBIC J. Biol. Inorg. Chem. 2012, 17, 399–407. [Google Scholar] [CrossRef]
- Qin, C.-L.; Tang, J.; Kim, K. Cloning and in vitro expression of TPK3, a Toxoplasma gondii homologue of shaggy/glycogen synthase kinase-3 kinases1. Mol. Biochem. Parasitol. 1998, 93, 273–283. [Google Scholar] [CrossRef]
- Fentress, S.J.; Behnke, M.S.; Dunay, I.R.; Mashayekhi, M.; Rommereim, L.M.; Fox, B.A.; Bzik, D.J.; Taylor, G.A.; Turk, B.E.; Lichti, C.F.; et al. Phosphorylation of Immunity-Related GTPases by a Toxoplasma gondii-Secreted Kinase Promotes Macrophage Survival and Virulence. Cell Host Microbe 2010, 8, 484–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.Z.; Thuraisingam, T.; Kanagaratham, C.; Tao, S.; Radzioch, D. c-Src kinase is involved in the tyrosine phosphorylation and activity of SLC11A1 in differentiating macrophages. PLoS ONE 2018, 13, e0196230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cable, J.; Prutzman, K.; Gunawardena, H.P.; Schaller, M.D.; Chen, X.; Campbell, S.L. In Vitro Phosphorylation of the Focal Adhesion Targeting Domain of Focal Adhesion Kinase by Src Kinase. Biochemistry 2012, 51, 2213–2223. [Google Scholar] [CrossRef] [Green Version]
- Samarasimhareddy, M.; Mayer, G.; Hurevich, M.; Friedler, A. Multiphosphorylated peptides: Importance, synthetic strategies, and applications for studying biological mechanisms. Org. Biomol. Chem. 2020, 18, 3405–3422. [Google Scholar] [CrossRef]
- Clark, A.R.; Dean, J.L.E.E. The control of inflammation via the phosphorylation and dephosphorylation of tristetraprolin: A tale of two phosphatases. Biochem. Soc. Trans. 2016, 44, 1321–1337. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Dzineku, F.; Blackshear, P.J. Expression and purification of recombinant tristetraprolin that can bind to tumor necrosis factor-α mRNA and serve as a substrate for mitogen-activated protein kinases. Arch. Biochem. Biophys. 2003, 412, 106–120. [Google Scholar] [CrossRef]
- Cao, H. Expression, Purification, and Biochemical Characterization of the Antiinflammatory Tristetraprolin: A Zinc-Dependent mRNA Binding Protein Affected by Posttranslational Modifications†,‡. Biochemistry 2004, 43, 13724–13738. [Google Scholar] [CrossRef] [Green Version]
- Erdem, F.A.; Salzer, I.; Heo, S.; Chen, W.; Jung, G.; Lubec, G.; Boehm, S.; Yang, J.-W. Updating In Vivo and In Vitro Phosphorylation and Methylation Sites of Voltage-Gated Kv7.2 Potassium Channels. Proteomics 2017, 17, 1700015. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Brena, B.; Batista-Viera, F. Methods in BiotechnologyTM. In Immobilization of Enzymes and Cells, 2nd ed.; Guisan, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2006; Volume 22, ISBN 978-1-58829-290-2. [Google Scholar]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, M.C.; Temporini, C.; Calleri, E.; Bruni, G.; Ducati, R.G.; Santos, D.S.; Cardoso, C.L.; Cass, Q.B.; Massolini, G. Evaluation of capillary chromatographic supports for immobilized human purine nucleoside phosphorylase in frontal affinity chromatography studies. J. Chromatogr. A 2014, 1338, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Ferreira, M.L. Chitosan-immobilized lipases for the catalysis of fatty acid esterifications. Enzym. Microb. Technol. 2007, 40, 769–777. [Google Scholar] [CrossRef]
- Narwal, V.; Pundir, C.S. Development of glycerol biosensor based on co-immobilization of enzyme nanoparticles onto graphene oxide nanoparticles decorated pencil graphite electrode. Int. J. Biol. Macromol. 2019, 127, 57–65. [Google Scholar] [CrossRef]
- Machín, B.; Chaves, S.; Ávila, C.; Pera, L.M.; Chehín, R.N.; Pingitore, E.V. Highly reusable invertase biocatalyst: Biological fibrils functionalized by photocrosslinking. Food Chem. 2020, 331, 127322. [Google Scholar] [CrossRef]
- Wang, L.; Wei, L.; Chen, Y.; Jiang, R. Specific and reversible immobilization of NADH oxidase on functionalized carbon nanotubes. J. Biotechnol. 2010, 150, 57–63. [Google Scholar] [CrossRef]
- Hetmann, A.; Wujak, M.; Bolibok, P.; Zięba, W.; Wiśniewski, M.; Roszek, K. Novel biocatalytic systems for maintaining the nucleotide balance based on adenylate kinase immobilized on carbon nanostructures. Mater. Sci. Eng. C 2018, 88, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Ding, M.; Sun, X.; Zhuang, W.; Liu, D.; Ying, H.; Zhu, C.; Chen, Y. Immobilization of a polyphosphate kinase 2 by coordinative self-assembly of his-tagged units with metal-organic frameworks and its application in ATP regeneration from AMP. Colloids Surf. B Biointerfaces 2019, 181, 261–269. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Zaitsev, S.Y.; Savina, A.A.; Zaitsev, I.S. Biochemical aspects of lipase immobilization at polysaccharides for biotechnology. Adv. Colloid Interface Sci. 2019, 272, 102016. [Google Scholar] [CrossRef]
- Rotková, J.; Šuláková, R.; Korecká, L.; Zdražilová, P.; Jandová, M.; Lenfeld, J.; Horák, D.; Bilkova, Z. Laccase immobilized on magnetic carriers for biotechnology applications. J. Magn. Magn. Mater. 2009, 321, 1335–1340. [Google Scholar] [CrossRef]
- Slovakova, M.; Minc, N.; Bilkova, Z.; Smadja, C.; Faigle, W.; Fütterer, C.; Taverna, M.; Viovy, J.-L. Use of self assembled magnetic beads for on-chip protein digestion. Lab. Chip 2005, 5, 935–942. [Google Scholar] [CrossRef]
- Ferey, J.; Da Silva, D.; Colas, C.; Nehmé, R.; Lafite, P.; Roy, V.; Morin, P.; Daniellou, R.; Agrofoglio, L.; Maunit, B. Monitoring of successive phosphorylations of thymidine using free and immobilized human nucleoside/nucleotide kinases by Flow Injection Analysis with High-Resolution Mass Spectrometry. Anal. Chim. Acta 2019, 1049, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Svitel, J.; Boukari, H.; Van Ryk, D.; Willson, R.C.; Schuck, P. Probing the Functional Heterogeneity of Surface Binding Sites by Analysis of Experimental Binding Traces and the Effect of Mass Transport Limitation. Biophys. J. 2007, 92, 1742–1758. [Google Scholar] [CrossRef] [Green Version]
- Merrill, A.H.; McCormick, D.B. Preparation and properties of immobilized flavokinase. Biotechnol. Bioeng. 1979, 21, 1629–1638. [Google Scholar] [CrossRef]
- Berke, W.; Schüz, H.-J.; Wandrey, C.; Morr, M.; Denda, G.; Kula, M.-R. Continuous regeneration of ATP in enzyme membrane reactor for enzymatic syntheses. Biotechnol. Bioeng. 1988, 32, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ropp, P.A.; Cheng, P.-W. Enzymatic synthesis of UDP-GlcN by a two step hollow fiber enzyme reactor system. Anal. Biochem. 1990, 187, 104–108. [Google Scholar] [CrossRef]
- Fonong, T. Immobilized enzyme assay of creatine kinase with amperometric detection. Anal. Biochem. 1989, 176, 234–238. [Google Scholar] [CrossRef]
- Ferey, J.; Da Silva, D.; Colas, C.; Lafite, P.; Topalis, D.; Roy, V.; Agrofoglio, L.A.; Daniellou, R.; Maunit, B. Monitoring of phosphorylation using immobilized kinases by on-line enzyme bioreactors hyphenated with High-Resolution Mass Spectrometry. Talanta 2019, 205, 120120. [Google Scholar] [CrossRef] [PubMed]
- Serra, I.; Conti, S.; Piškur, J.; Clausen, A.R.; Terreni, M.; Ubiali, D.; Munch-Petersen, B. Immobilized Drosophila melanogaster Deoxyribonucleoside Kinase (DmdNK) as a High Performing Biocatalyst for the Synthesis of Purine Arabinonucleotides. Adv. Synth. Catal. 2014, 356, 563–570. [Google Scholar] [CrossRef]
- Wu, S.; Li, J.; Hu, C.; Tian, J.; Zhang, T.; Chen, N.; Fan, X. Concomitant use of immobilized uridine-cytidine kinase and polyphosphate kinase for 5′-cytidine monophosphate production. Chin. J. Biotechnol 2020, 36, 1002–1011. [Google Scholar] [CrossRef]
- Hoffman, R.C., Jr.; Wyman, P.L.; Smith, L.E.; Nolt, C.L.; Conley, J.L.; Hevel, J.M.; Warren, J.P.; Reiner, G.A.; Moe, O.A., Jr. Immobilized polyphosphate kinase: Preparation, properties, and potential for use in adenosine 5′-triphosphate regeneration. Biotechnol. Appl. Biochem. 1988, 10, 107–117. [Google Scholar] [CrossRef]
- Suzuki, S.; Hara, R.; Kino, K. Production of aminoacyl prolines using the adenylation domain of nonribosomal peptide synthetase with class III polyphosphate kinase 2-mediated ATP regeneration. J. Biosci. Bioeng. 2018, 125, 644–648. [Google Scholar] [CrossRef]
- Sun, X.; Niu, H.; Song, J.; Jiang, D.; Leng, J.; Zhuang, W.; Chen, Y.; Liu, D.; Ying, H. Preparation of a Copper Polyphosphate Kinase Hybrid Nanoflower and Its Application in ADP Regeneration from AMP. ACS Omega 2020, 5, 9991–9998. [Google Scholar] [CrossRef]
- Kowalczyk, T.H.; Szymona, O. Glucose determination using immobilized polyphosphate glucokinase. Anal. Biochem. 1991, 197, 326–332. [Google Scholar] [CrossRef]
- Gabris, C.; Bengelsdorf, F.R.; Dürre, P. Analysis of the key enzymes of butyric and acetic acid fermentation in biogas reactors. Microb. Biotechnol. 2015, 8, 865–873. [Google Scholar] [CrossRef]
- Castro, L.B.R.; Silva, F.F.; Carmona-Ribeiro, A.M.; Kappl, M.; Petri, D.F.S. Immobilization of Hexokinase onto Chitosan Decorated Particles†. J. Phys. Chem. B 2007, 111, 8520–8526. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Barford, J.P.; Renneberg, R. Amperometric trienzyme ATP biosensors based on the coimmobilization of salicylate hydroxylase, glucose-6-phosphate dehydrogenase, and hexokinase. Sensors Actuators B Chem. 2008, 132, 1–4. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Kucherenko, D.Y.; Soldatkin, O.O.; Lagarde, F.; Dzyadevych, S.V.; Soldatkin, A.P. A novel conductometric biosensor based on hexokinase for determination of adenosine triphosphate. Talanta 2016, 150, 469–475. [Google Scholar] [CrossRef]
- Narwal, V.; Pundir, C.S. Fabrication of glycerol biosensor based on co-immobilization of enzyme nanoparticles onto pencil graphite electrode. Anal. Biochem. 2018, 555, 94–103. [Google Scholar] [CrossRef]
- Chauhan, N.; Narang, J.; Pundir, C.S. Covalent immobilization of lipase, glycerol kinase, glycerol-3-phosphate oxidase & horseradish peroxidase onto plasticized polyvinyl chloride (PVC) strip & its application in serum triglyceride determination. Indian J. Med. Res. 2014, 139, 603–609. [Google Scholar]
- Minakshi, C.S.; Pundir, C.S. Co-immobilization of lipase, glycerol kinase, glycerol-3-phosphate oxidase and peroxidase on to aryl amine glass beads affixed on plastic strip for determination of triglycerides in serum. Indian J. Biochem. Biophys. 2008, 45, 111–115. [Google Scholar]
- Kalia, V.; Pundir, C.S. Co-immobilization of lipase, glycerol kinase, glycerol-3-phosphate oxidase and peroxidase onto alkylamine glass beads through glutaraldehyde coupling. Indian J. Biochem. Biophys. 2002, 39, 342–346. [Google Scholar] [PubMed]
- Pundir, C.S.; Aggarwal, V. Amperometric triglyceride bionanosensor based on nanoparticles of lipase, glycerol kinase, glycerol-3-phosphate oxidase. Anal. Biochem. 2017, 517, 56–63. [Google Scholar] [CrossRef]
- Taka’aki, M.; Kenischi, M.; Takuya, M.; Tetsu, M.; Masateru, O.; Yasuhiko, S. High-Throughput Profiling of Kinase Inhibitors Selectivity Using the ProteOnTM XPR36 Protein Interaction Array System. Available online: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_5960A.pdf (accessed on 11 March 2021).
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Popplewell, J.; Luo, R. Kinase Immobilization for Small Molecule Inhibition Studies. Bioradiations Articles. Available online: https://www.bioradiations.com/immobilization-of-active-kinases-for-small-molecule-inhibition-studies/ (accessed on 11 March 2021).
- Zhou, Y.; Guo, T.; Tang, G.; Wu, H.; Wong, N.-K.; Pan, Z. Site-Selective Protein Immobilization by Covalent Modification of GST Fusion Proteins. Bioconjug. Chem. 2014, 25, 1911–1915. [Google Scholar] [CrossRef]
- Wang, X.; Guo, T.; Chen, J.; Li, X.; Zhou, Y.; Pan, Z. Covalent and selective immobilization of GST fusion proteins with fluorophosphonate-based probes. Chem. Commun. 2018, 54, 4661–4664. [Google Scholar] [CrossRef]
- Kim, M.; Shin, D.-S.; Kim, J.; Lee, Y.-S. Substrate screening of protein kinases: Detection methods and combinatorial peptide libraries. Biopolymers 2010, 94, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Liao, P.-C. Analysis of protein phosphorylation using mass spectrometry. Chang. Gung Med. J. 2008, 31, 217–227. [Google Scholar]
- Campbell, D.G.; Morrice, N.A. Identification of protein phosphorylation sites by a combination of mass spectrometry and solid phase Edman sequencing. J. Biomol. Tech. JBT 2002, 13, 119–130. [Google Scholar] [PubMed]
- Molden, R.C.; Goya, J.; Khan, Z.; Garcia, B.A. Stable Isotope Labeling of Phosphoproteins for Large-scale Phosphorylation Rate Determination. Mol. Cell. Proteom. 2014, 13, 1106–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Protein Kinase | Solid Support | Method of Immobilization and Purpose | Ref. |
|---|---|---|---|
| Biotinylated kinases | NeutrAvidin surface sensor chip | Biotin-avidin affinity bond for high-throughput profiling of kinase inhibitor selectivity | [126,128] |
| ITK, EGFR, BLK, and LCK recombinant, GST-tagged | CH-sepharose 4B beads; | Site selective covalent kinase immobilization | [129,130] |
| GSK-3β, GST-tagged recombinant | Glutathione beads, magnetic; | Affinity kinase immobilization for kinase inhibitors screening | [60] |
| GSK-3β, recombinant; ERK2/MAPK1 recombinant, active | SeraMag SpeedBeads, carboxyl; 0.816 μm; magnetic BcMag, aldehyde; magnetic | Covalent kinase immobilization for in vitro phosphorylation | [59] |
| GSK-3β His-tagged, recombinant, MAPK2 His-tagged, recombinant | SIMAG-IDA/Co3+, SIMAG-IDA/Ni2+ magnetic; | Affinity irreversible kinase immobilization for in vitro phosphorylation | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slovakova, M.; Bilkova, Z. Contemporary Enzyme-Based Methods for Recombinant Proteins In Vitro Phosphorylation. Catalysts 2021, 11, 1007. https://doi.org/10.3390/catal11081007
Slovakova M, Bilkova Z. Contemporary Enzyme-Based Methods for Recombinant Proteins In Vitro Phosphorylation. Catalysts. 2021; 11(8):1007. https://doi.org/10.3390/catal11081007
Chicago/Turabian StyleSlovakova, Marcela, and Zuzana Bilkova. 2021. "Contemporary Enzyme-Based Methods for Recombinant Proteins In Vitro Phosphorylation" Catalysts 11, no. 8: 1007. https://doi.org/10.3390/catal11081007
APA StyleSlovakova, M., & Bilkova, Z. (2021). Contemporary Enzyme-Based Methods for Recombinant Proteins In Vitro Phosphorylation. Catalysts, 11(8), 1007. https://doi.org/10.3390/catal11081007