The Promotor and Poison Effects of the Inorganic Elements of Kraft Lignin during Hydrotreatment over NiMoS Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lignin Characterization
2.2. Catalytic Activity during Hydrotreatment
2.3. Recycle Studies
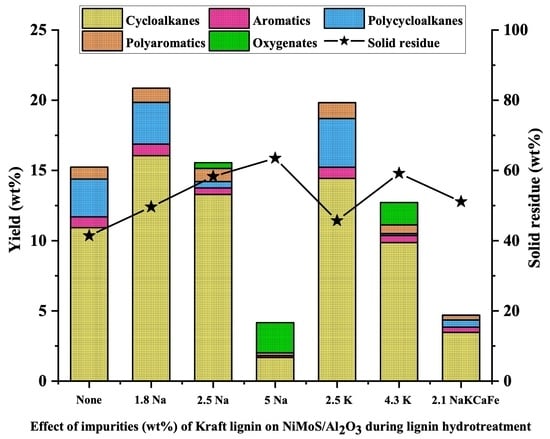
2.4. Effect of the Amount of Na on the Activity of NiMoS/Al2O3 Catalyst
2.5. Effect of Other Impurities on the Activity of NiMoS/Al2O3
2.6. The Chemical Nature of Na in Kraft Lignin
2.7. Pretreatment of Kraft Lignin
2.7.1. Characterization of Pretreated Lignin
2.7.2. Hydrotreatment of Pretreated Lignin
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, P.; Abdelaziz, O.Y.; Hulteberg, C.P.; Riisager, A. New synthetic approaches to biofuels from lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2020, 21, 16–21. [Google Scholar] [CrossRef]
- Walch, F.; Abdelaziz, O.Y.; Meier, S.; Bjelić, S.; Hulteberg, C.P.; Riisager, A. Oxidative depolymerization of Kraft lignin to high-value aromatics using a homogeneous vanadium–copper catalyst. Catal. Sci. Technol. 2021, 11, 1843–1853. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shuai, L.; Kim, H.; Motagamwala, A.H.; Mobley, J.K.; Yue, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Chen, F.; Dixon, R.A.; et al. An “ideal lignin” facilitates full biomass utilization. Sci. Adv. 2018, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, T.W.; Motagamwala, A.H.; Dumesic, J.A.; Huber, G.W. Fundamental catalytic challenges to design improved biomass conversion technologies. J. Catal. 2019, 369, 518–525. [Google Scholar] [CrossRef]
- McClelland, D.J.; Galebach, P.H.; Motagamwala, A.H.; Wittrig, A.M.; Karlen, S.D.; Buchanan, J.S.; Dumesic, J.A.; Huber, G.W. Supercritical methanol depolymerization and hydrodeoxygenation of lignin and biomass over reduced copper porous metal oxides. Green Chem. 2019, 21, 2988–3005. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Chen, H. 3-Lignocellulose biorefinery feedstock engineering. In Lignocellulose Biorefinery Engineering; Chen, H., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 37–86. [Google Scholar]
- Bruijnincx, P.; Weckhuysen, B.; Gruter, G.-J.; Engelen-Smeets, E. Lignin Valorisation: The Importance of a Full Value Chain Approach; Utrecht University: Utrecht, The Netherlands, 2016. [Google Scholar]
- Dessbesell, L.; Yuan, Z.; Leitch, M.; Paleologou, M.; Pulkki, R.; Xu, C.C. Capacity design of a kraft lignin biorefinery for production of biophenol via a proprietary low-temperature/low-pressure lignin depolymerization process. ACS Sustain. Chem. Eng. 2018, 6, 9293–9303. [Google Scholar] [CrossRef]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery review: Wide-reaching products through kraft lignin. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Lamb, A.C.; Lee, A.F.; Wilson, K. Recent Advances in Heterogeneous Catalyst Design for Biorefining. Aust. J. Chem. 2020, 73, 832–852. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdari, R.K.; Agarwal, S.; Heeres, H.J. Hydrotreatment of Kraft Lignin to Alkylphenolics and Aromatics Using Ni, Mo, and W Phosphides Supported on Activated Carbon. ACS Sustain. Chem. Eng. 2019, 7, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- Horáček, J.; Homola, F.; Kubičková, I.; Kubička, D. Lignin to liquids over sulfided catalysts. Catal. Today 2012, 179, 191–198. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, L.; Gu, J.; Gou, L.; Xie, L.; Wang, Y.; Dai, L. Catalytic hydrotreatment of kraft lignin into aromatic alcohols over nickel-rhenium supported on niobium oxide catalyst. Bioresour. Technol. 2020, 299, 122582. [Google Scholar] [CrossRef] [PubMed]
- Narani, A.; Chowdari, R.K.; Cannilla, C.; Bonura, G.; Frusteri, F.; Heeres, H.J.; Barta, K. Efficient catalytic hydrotreatment of Kraft lignin to alkylphenolics using supported NiW and NiMo catalysts in supercritical methanol. Green Chem. 2015, 17, 5046–5057. [Google Scholar] [CrossRef] [Green Version]
- Dou, X.; Jiang, X.; Li, W.; Zhu, C.; Liu, Q.; Lu, Q.; Zheng, X.; Chang, H.-m.; Jameel, H. Highly efficient conversion of Kraft lignin into liquid fuels with a Co-Zn-beta zeolite catalyst. Appl. Catal. B Environ. 2020, 268, 118429. [Google Scholar] [CrossRef]
- Chen, M.; Lu, H.; Wang, Y.; Tang, Z.; Zhang, J.; Wang, C.; Yang, Z.; Wang, J.; Zhang, H. Effect of Reduction Treatments of Mo/Sepiolite Catalyst on Lignin Depolymerization under Supercritical Ethanol. Energy Fuels 2020, 34, 3394–3405. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Elliott, D.C.; Peterson, K.L.; Muzatko, D.S.; Alderson, E.V.; Hart, T.R.; Neuenschwander, G.G. Effects of trace contaminants on catalytic processing of biomass-derived feedstocks. Appl. Biochem. Biotechnol. 2004, 115, 807–825. [Google Scholar] [CrossRef]
- Stummann, M.Z.; Høj, M.; Hansen, A.B.; Beato, P.; Wiwel, P.; Gabrielsen, J.; Jensen, P.A.; Jensen, A.D. Deactivation of a CoMo Catalyst during Catalytic Hydropyrolysis of Biomass. Part 1. Product Distribution and Composition. Energy Fuels 2019, 33, 12374–12386. [Google Scholar] [CrossRef]
- Stummann, M.Z.; Høj, M.; Davidsen, B.; Hansen, L.P.; Beato, P.; Gabrielsen, J.; Jensen, P.A.; Jensen, A.D. Deactivation of a CoMo Catalyst during Catalytic Hydropyrolysis of Biomass. Part 2. Characterization of the Spent Catalysts and Char. Energy Fuels 2019, 33, 12387–12402. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Fidalgo, B. 15-Production of bio-syngas and bio-hydrogen via gasification. In Handbook of Biofuels Production, 2nd ed.; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Duxford, UK, 2016; pp. 431–494. [Google Scholar]
- Patton, R.; Steele, P.; Yu, F. Coal vs. Charcoal-fueled Diesel Engines: A Review. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 315–322. [Google Scholar] [CrossRef]
- Munick de Albuquerque Fragoso, D.; Bouxin, F.P.; Montgomery, J.R.D.; Westwood, N.J.; Jackson, S.D. Catalytic depolymerisation of isolated lignin to fine chemicals: Depolymerisation of Kraft lignin. Bioresour. Technol. Rep. 2020, 9, 100400. [Google Scholar] [CrossRef]
- Joffres, B.; Nguyen, M.T.; Laurenti, D.; Lorentz, C.; Souchon, V.; Charon, N.; Daudin, A.; Quignard, A.; Geantet, C. Lignin hydroconversion on MoS2-based supported catalyst: Comprehensive analysis of products and reaction scheme. Appl. Catal. B Environ. 2016, 184, 153–162. [Google Scholar] [CrossRef]
- Nakadi, F.V.; Prodanov, C.; Boschetti, W.; Vale, M.G.R.; Welz, B.; de Andrade, J.B. Determination of silicon in biomass and products of pyrolysis process via high-resolution continuum source atomic absorption spectrometry. Talanta 2018, 179, 828–835. [Google Scholar] [CrossRef]
- Wu, J.-W.; Shi, Y.; Zhu, Y.-X.; Wang, Y.-C.; Gong, H.-J. Mechanisms of Enhanced Heavy Metal Tolerance in Plants by Silicon: A Review. Pedosphere 2013, 23, 815–825. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; Guan, Y. Excellent Adsorption–Desorption of Ammonium by a Poly(acrylic acid)-Grafted Chitosan and Biochar Composite for Sustainable Agricultural Development. ACS Sustain. Chem. Eng. 2020, 8, 16451–16462. [Google Scholar] [CrossRef]
- Gupta, S.; Mondal, P.; Borugadda, V.B.; Dalai, A.K. Advances in upgradation of pyrolysis bio-oil and biochar towards improvement in bio-refinery economics: A comprehensive review. Environ. Technol. Innov. 2021, 21, 101276. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Tavasoli, A.; Dalai, A.K. Synthesis of higher alcohols from syngas over alkali promoted MoS2 catalysts supported on multi-walled carbon nanotubes. Appl. Catal. A Gen. 2009, 365, 243–251. [Google Scholar] [CrossRef]
- Brauckmann, J.O.; Verhoef, R.; Schotman, A.H.M.; Kentgens, A.P.M. Solid-State Nuclear Magnetic Resonance Characterization of Residual 23Na in Aramid Fibers. J. Phys. Chem. C 2019, 123, 14439–14448. [Google Scholar] [CrossRef] [Green Version]
- Verhulst, H.A.M.; Welters, W.; Vorbeck, G.; Ljm, V.; Vhj, B.; van Santen, R.; Haan, J. A new assignment of the signals in 23Na DOR NMR to sodium sites in dehydrated Na Y Zeolite. J. Phys. Chem. 1994, 98, 639–7062. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, E.M.; Root, T.W.; Cooper, S.L. Morphological studies of lightly-sulfonated polystyrene using 23Na NMR. 1. Effects of sample composition. Macromolecules 1994, 27, 5803–5810. [Google Scholar] [CrossRef]
- Persson, H.; Kantarelis, E.; Evangelopoulos, P.; Yang, W. Wood-derived acid leaching of biomass for enhanced production of sugars and sugar derivatives during pyrolysis: Influence of acidity and treatment time. J. Anal. Appl. Pyrolysis 2017, 127, 329–334. [Google Scholar] [CrossRef]
- Klett, A.S.; Payne, A.M.; Thies, M.C. Continuous-Flow Process for the Purification and Fractionation of Alkali and Organosolv Lignins. ACS Sustain. Chem. Eng. 2016, 4, 6689–6694. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Yoosuk, B.; Sanggam, P.; Wiengket, S.; Prasassarakich, P. Hydrodeoxygenation of oleic acid and palmitic acid to hydrocarbon-like biofuel over unsupported Ni-Mo and Co-Mo sulfide catalysts. Renew. Energy 2019, 139, 1391–1399. [Google Scholar] [CrossRef]
- Baharudin, K.B.; Taufiq-Yap, Y.H.; Hunns, J.; Isaacs, M.; Wilson, K.; Derawi, D. Mesoporous NiO/Al-SBA-15 catalysts for solvent-free deoxygenation of palm fatty acid distillate. Microporous Mesoporous Mater. 2019, 276, 13–22. [Google Scholar] [CrossRef]
- Arora, P.; Abdolahi, H.; Cheah, Y.W.; Salam, M.A.; Grennfelt, E.L.; Rådberg, H.; Creaser, D.; Olsson, L. The role of catalyst poisons during hydrodeoxygenation of renewable oils. Catal. Today 2021, 367, 28–42. [Google Scholar] [CrossRef]
- Badoga, S.; Alvarez-Majmutov, A.; Xing, T.; Gieleciak, R.; Chen, J. Co-processing of Hydrothermal Liquefaction Biocrude with Vacuum Gas Oil through Hydrotreating and Hydrocracking to Produce Low-Carbon Fuels. Energy Fuels 2020, 34, 7160–7169. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Korneeva, E.V.; Bukhtiyarova, G.A.; Nuzhdin, A.L.; Budneva, A.A.; Prosvirin, I.P.; Zaikovskii, V.I.; Noskov, A.S. Hydrocracking of vacuum gas oil in the presence of supported nickel-tungsten catalysts. Kinet. Catal. 2011, 52, 446–458. [Google Scholar] [CrossRef]







| Elements a | Kraft Lignin (ppm) | Pretreated Kraft Lignin (ppm) |
|---|---|---|
| S | 21,000 | 9000 |
| Na | 9300 | 28 |
| Si | 6000 | <100 |
| K | 1100 | 5 |
| Ca | 200 | 7 |
| Mn | 58 | 1.7 |
| Fe | 30 | 24 |
| B | 22 | 4 |
| Mg | 21 | 9 |
| Al | 18 | 125 |
| Element | Fresh Catalyst (ppm) | After 3rd Run (ppm) | Solid Residue (ppm) |
|---|---|---|---|
| Ni | 38,500 | 38,500 | 72 |
| Mo | 111,000 | 104,000 | 90 |
| Na | <50 | 357 | 15,400 |
| Ca | <50 | 180 | 600 |
| K | <30 | 180 | 1550 |
| Fe | 114 | 183 | 233 |
| Si | <200 | <200 | <500 |
| Mn | 10.5 | 15.4 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastian, J.; Cheah, Y.W.; Bernin, D.; Creaser, D.; Olsson, L. The Promotor and Poison Effects of the Inorganic Elements of Kraft Lignin during Hydrotreatment over NiMoS Catalyst. Catalysts 2021, 11, 874. https://doi.org/10.3390/catal11080874
Sebastian J, Cheah YW, Bernin D, Creaser D, Olsson L. The Promotor and Poison Effects of the Inorganic Elements of Kraft Lignin during Hydrotreatment over NiMoS Catalyst. Catalysts. 2021; 11(8):874. https://doi.org/10.3390/catal11080874
Chicago/Turabian StyleSebastian, Joby, You Wayne Cheah, Diana Bernin, Derek Creaser, and Louise Olsson. 2021. "The Promotor and Poison Effects of the Inorganic Elements of Kraft Lignin during Hydrotreatment over NiMoS Catalyst" Catalysts 11, no. 8: 874. https://doi.org/10.3390/catal11080874
APA StyleSebastian, J., Cheah, Y. W., Bernin, D., Creaser, D., & Olsson, L. (2021). The Promotor and Poison Effects of the Inorganic Elements of Kraft Lignin during Hydrotreatment over NiMoS Catalyst. Catalysts, 11(8), 874. https://doi.org/10.3390/catal11080874