Green Synthesis of Metallic Nanoparticles: Applications and Limitations
Abstract
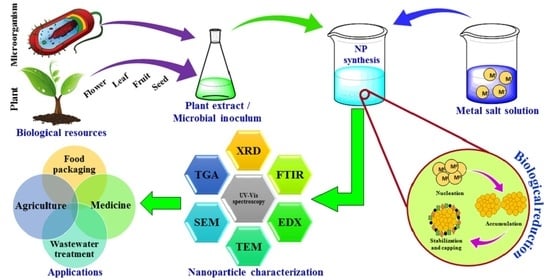
:1. Introduction
2. Biological Synthesis of NPs
2.1. Plant-Mediated Synthesis of NPs
2.2. Microbial Synthesis of NPs
2.3. Factors Affecting NPs Synthesis
2.4. Characterization of NPs
3. Potential Applications of Metal NPs
3.1. Agriculture
3.1.1. Effect of Nano Material on Plants
3.1.2. Application of Nanomaterials in the Field of Agriculture
Nanofertilizers
Nanopesticide
3.2. Nanoparticles in Food Industry
3.2.1. Application of NPs in Food Preservation and Packaging
3.2.2. Applications of NPs in Food Supplements and Value Addition
3.3. Drug and Medicine
3.3.1. Silver NPs (AgNPs)
3.3.2. Gold NPs (AuNPs)
3.3.3. Iron Oxide NPs
3.4. Wastewater Treatment Process
3.5. Antimicrobial Activity
4. Toxicity of Metal Nanoparticles
4.1. Impact of Nanoparticle Toxicity on Plants
Assessment of NPs Phytotoxic and Genotoxic Effects on the Plants
4.2. Toxicity of Nanoparticle-Based Drugs
5. Major Challenges and Future Perspective
- Detailed optimization studies on reactants (plant extract, microorganism inoculum, fermentation medium composition, etc.) and process parameters (temperature, pH, rotational speed, etc.) are required to control the size and shape of the NPs.
- Studies also need to be focused on enhancing various physicochemical characteristics of NPs for specific applications.
- The involvement of each metabolite of plant extract and cellular components of microorganism in the synthesis of NPs should be completely analyzed.
- Scale-up of NPs production for commercial purposes using green synthesis methods needs to be prioritized.
- Improvement of NPs yield and stability with reduced reaction time is needed by optimizing various reaction parameters.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current applications and future scope in food. Food Front. 2021, 2, 3–22. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Park, J.Y.; Kwon, C.W.; Hong, S.C.; Park, K.M.; Chang, P.S. An overview of nanotechnology in food science: Preparative methods, practical applications, and safety. J. Chem. 2018, 2018, 5427978. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385. [Google Scholar] [PubMed]
- Banne, S.V.; Patil, M.S.; Kulkarni, R.M.; Patil, S.J. Synthesis and characterization of silver nano particles for EDM applications. Mater. Today 2017, 4, 12054–12060. [Google Scholar] [CrossRef]
- Tan, Y.; Dai, X.; Li, Y.; Zhu, D. Preparation of gold, platinum, palladium and silver nanoparticles by the reduction of their salts with a weak reductant-potassium bitartrate. J. Mater. Chem. 2003, 13, 1069–1075. [Google Scholar] [CrossRef]
- Norris, C.B.; Joseph, P.R.; Mackiewicz, M.R.; Reed, S.M. Minimizing formaldehyde use in the synthesis of gold− silver core− shell nanoparticles. Chem. Mater. 2010, 22, 3637–3645. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Polymer stabilized silver nanoparticles: A photochemical synthesis route. J. Mater. Sci. 2004, 39, 4459–4463. [Google Scholar] [CrossRef]
- Eluri, R.; Paul, B. Synthesis of nickel nanoparticles by hydrazine reduction: Mechanistic study and continuous flow synthesis. J. Nanopart. Res. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Dehghani, H. Sodium-dodecyl-sulphate-assisted synthesis of Ni nanoparticles: Electrochemical properties. Bull. Mater. Sci. 2017, 40, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Pandey, G.; Singh, S.; Hitkari, G. Synthesis and characterization of polyvinyl pyrrolidone (PVP)-coated Fe3O4 nanoparticles by chemical co-precipitation method and removal of Congo red dye by adsorption process. Int. Nano Lett. 2018, 8, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Xie, H. Nanoparticles in daily life: Applications, toxicity and regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Hua, S.; De Matos, M.B.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Sriramulu, M.; Shanmugam, S.; Ponnusamy, V.K. Agaricus bisporus mediated biosynthesis of copper nanoparticles and its biological effects: An in-vitro study. Colloid Interface Sci. Commun. 2020, 35, 100254. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv. Colloid Interfac. 2011, 169, 59–79. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Parsons, J.G.; Gomez, E.; Peralta-Videa, J.; Troiani, H.E.; Santiago, P.; Yacaman, M.J. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Bali, R.; Harris, A.T. Biogenic synthesis of Au nanoparticles using vascular plants. Ind. Eng. Chem. Res. 2010, 49, 12762–12772. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Adv. Mat. Res. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Muthukumaran, P.; Ramachandran, R.; Balakumaran, M.D.; Kalaichelvan, P.T. Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol. Rep. 2014, 4, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus Rosa Sinensis. Phys. E 2010, 42, 1417–1424. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Ranjbar, S.; Gupta, V.K.; Asif, M.; Pourseyedi, S.; Karimi, M.J.; Mohammadinejad, R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015, 207, 159–163. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.N.; Kim, J.H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Pragathiswaran, C.; Violetmary, J.; Faritha, A.; Selvarani, K.; Nawas, P.M. Photocatalytic degradation, sensing of Cd2+ using silver nanoparticles synthesised from plant extract of Cissus quadrangularis and their microbial activity. Mater. Today Proc. 2021, 45, 3348–3356. [Google Scholar] [CrossRef]
- Salih, T.A.; Hassan, K.T.; Majeed, S.R.; Ibraheem, I.J.; Hassan, O.M.; Obaid, A.S. In vitro scolicidal activity of synthesised silver nanoparticles from aqueous plant extract against Echinococcus granulosus. Biotechnol. Rep. 2020, 28, e00545. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.A.; Chowdhury, R.A.; Das, S.; Nahian, M.K.; Islam, D.; Gafur, M.A. Plant-mediated green synthesis and characterization of silver nanoparticles using Phyllanthus emblica fruit extract. Mater. Today Proc. 2021, 42, 1867–1871. [Google Scholar] [CrossRef]
- Chavan, R.R.; Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Urade, M.N. Characterization, antioxidant, antimicrobial and cytotoxic activities of green synthesized silver and iron nanoparticles using alcoholic Blumea eriantha DC plant extract. Mater. Today Commun. 2020, 24, 101320. [Google Scholar] [CrossRef]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.M.I.; Krause, R.W.; Memvanga, P.B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef]
- Vignesh, V.; Anbarasi, K.F.; Karthikeyeni, S.; Sathiyanarayanan, G.; Subramanian, P.; Thirumurugan, R. A superficial phyto-assisted synthesis of silver nanoparticles and their assessment on hematological and biochemical parameters in Labeo rohita (Hamilton, 1822). Colloids Surf. A 2013, 439, 184–192. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, X.; Zhang, Y.; Xue, X.; Fu, Y. Biosynthesis of silver nanoparticles at room temperature using aqueous aloe leaf extract and antibacterial properties. Colloids Surf. A 2013, 423, 63–68. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crops Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef]
- Suriyakalaa, U.; Antony, J.J.; Suganya, S.; Siva, D.; Sukirtha, R.; Kamalakkannan, S.; Pichiah, P.T.; Achiraman, S. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf. B 2013, 102, 189–194. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; MubarakAli, D.; Sathishkumar, G.; Sivanandhan, G.; Dev, G.K.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N.; et al. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf. B 2013, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Bhaumik, J.; Kumar, S.; Banerjee, U.C. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid Interface Sci. 2014, 415, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Adewale, O.B.; Egbeyemi, K.A.; Onwuelu, J.O.; Potts-Johnson, S.S.; Anadozie, S.O.; Fadaka, A.O.; Osukoya, O.A.; Aluko, B.T.; Johnson, J.; Obafemi, T.O.; et al. Biological synthesis of gold and silver nanoparticles using leaf extracts of Crassocephalum rubens and their comparative in vitro antioxidant activities. Heliyon 2020, 6, e05501. [Google Scholar] [CrossRef]
- Daisy, P.; Saipriya, K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomed. 2012, 7, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangamani, N.; Bhuvaneshwari, N. Green synthesis of gold nanoparticles using Simarouba glauca leaf extract and their biological activity of micro-organism. Chem. Phys. Lett. 2019, 732, 136587. [Google Scholar] [CrossRef]
- Satpathy, S.; Patra, A.; Ahirwar, B.; Hussain, M.D. Process optimization for green synthesis of gold nanoparticles mediated by extract of Hygrophila spinosa T. Anders and their biological applications. Phys. E 2020, 121, 113830. [Google Scholar] [CrossRef]
- Kumar, P.V.; Kala, S.M.J.; Prakash, K.S. Green synthesis of gold nanoparticles using Croton Caudatus Geisel leaf extract and their biological studies. Mater. Lett. 2019, 236, 19–22. [Google Scholar] [CrossRef]
- Anand, K.; Gengan, R.M.; Phulukdaree, A.; Chuturgoon, A. Agroforestry waste Moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti-cancer and catalytic activity. J. Ind. Eng. Chem. 2015, 21, 1105–1111. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Pavagadhi, S.; Mahadevan, A.; Balasubramanian, R. Biosynthesis of gold nanoparticles and related cytotoxicity evaluation using A549 cells. Ecotox. Environ. Saf. 2015, 114, 232–240. [Google Scholar] [CrossRef]
- Gopinath, K.; Venkatesh, K.S.; Ilangovan, R.; Sankaranarayanan, K.; Arumugam, A. Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Ind. Crops Prod. 2013, 50, 737–742. [Google Scholar] [CrossRef]
- Kumar, K.P.; Paul, W.; Sharma, C.P. Green synthesis of gold nanoparticles with Zingiber officinale extract: Characterization and blood compatibility. Process Biochem. 2011, 46, 2007–2013. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Khoshnevisan, K.; Davoodi, D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.M.; Mandal, B.K.; Sinha, M.; Krishnakumar, V. Terminalia chebula mediated green and rapid synthesis of gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 86, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, W.; Yang, F.; Liu, H. Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 73–79. [Google Scholar] [CrossRef]
- Ganesan, R.M.; Prabu, H.G. Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications. Arab. J. Chem. 2019, 12, 2166–2174. [Google Scholar] [CrossRef] [Green Version]
- Muniyappan, N.; Nagarajan, N.S. Green synthesis of gold nanoparticles using Curcuma pseudomontana essential oil, its biological activity and cytotoxicity against human ductal breast carcinoma cells T47D. J. Environ. Chem. Eng. 2014, 2, 2037–2044. [Google Scholar] [CrossRef]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 15–23. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki-Miyaura coupling in water. J. Mol. Catal. A Chem. 2015, 396, 297–303. [Google Scholar] [CrossRef]
- Khazaei, A.; Rahmati, S.; Hekmatian, Z.; Saeednia, S. A green approach for the synthesis of palladium nanoparticles supported on pectin: Application as a catalyst for solvent-free Mizoroki-Heck reaction. J. Mol. Catal. A Chem. 2013, 372, 160–166. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of palladium nanoparticles. Mater. Lett. 2010, 64, 1951–1953. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Wang, H.; Huang, J.; Lin, L.; Wang, W.; Sun, D.; Su, Y.; Opiyo, J.B.; Hong, L.; et al. Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J. Nanoparticle Res. 2010, 12, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Kalaiselvi, A.; Roopan, S.M.; Madhumitha, G.; Ramalingam, C.; Elango, G. Synthesis and characterization of palladium nanoparticles using Catharanthus roseus leaf extract and its application in the photo-catalytic degradation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.M.; Mandal, B.K.; Kumar, K.S.; Reddy, P.S.; Sreedhar, B. Biobased green method to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Bagherzadeh, M.; Kiani, M.; Ghadiri, A.M. Rosmarinus officinalis directed palladium nanoparticle synthesis: Investigation of potential anti-bacterial, anti-fungal and Mizoroki-Heck catalytic activities. Adv. Powder Technol. 2020, 31, 1402–1411. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Green synthesis of palladium nanoparticles using gum ghatti (Anogeissus latifolia) and its application as an antioxidant and catalyst. Arab. J. Chem. 2018, 11, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Joseph Kirubaharan, C.; Fang, Z.; Sha, C.; Yong, Y.C. Green synthesis of Ag and Pd nanoparticles for water pollutants treatment. Water Sci. Technol. 2020, 82, 2344–2352. [Google Scholar] [CrossRef]
- Lebaschi, S.; Hekmati, M.; Veisi, H. Green synthesis of palladium nanoparticles mediated by black tea leaves (Camellia sinensis) extract: Catalytic activity in the reduction of 4-nitrophenol and Suzuki-Miyaura coupling reaction under ligand-free conditions. J. Colloid Interface Sci. 2017, 485, 223–231. [Google Scholar] [CrossRef]
- Sheny, D.S.; Philip, D.; Mathew, J. Synthesis of platinum nanoparticles using dried Anacardium occidentale leaf and its catalytic and thermal applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Kong, T.; Jing, X.; Odoom-Wubah, T.; Li, X.; Sun, D.; Lu, F.; Zheng, Y.; Huang, J.; Li, Q. Plant-mediated synthesis of platinum nanoparticles and its bioreductive mechanism. J. Colloid Interface Sci. 2013, 396, 138–145. [Google Scholar] [CrossRef]
- Raut, R.W.; Haroon, A.S.M.; Malghe, Y.S.; Nikam, B.T.; Kashid, S.B. Rapid biosynthesis of platinum and palladium metal nanoparticles using root extract of Asparagus racemosus Linn. Adv. Mater. Lett. 2013, 4, 650–654. [Google Scholar] [CrossRef]
- Song, J.Y.; Kwon, E.Y.; Kim, B.S. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioproc. Biosyst. Eng. 2010, 33, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Soundarrajan, C.; Sankari, A.; Dhandapani, P.; Maruthamuthu, S.; Ravichandran, S.; Sozhan, G.; Palaniswamy, N. Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioproc. Biosyst. Eng. 2012, 35, 827–833. [Google Scholar] [CrossRef]
- Razavi, R.; Molaei, R.; Moradi, M.; Tajik, H.; Ezati, P.; Yordshahi, A.S. Biosynthesis of metallic nanoparticles using mulberry fruit (Morus alba L.) extract for the preparation of antimicrobial nanocellulose film. Appl. Nanosci. 2020, 10, 465–476. [Google Scholar] [CrossRef]
- Lotha, R.; Shamprasad, B.R.; Sundaramoorthy, N.S.; Nagarajan, S.; Sivasubramanian, A. Biogenic phytochemicals (cassinopin and isoquercetin) capped copper nanoparticles (ISQ/CAS@ CuNPs) inhibits MRSA biofilms. Microb. Pathog. 2019, 132, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Khani, R.; Roostaei, B.; Bagherzade, G.; Moudi, M. Green synthesis of copper nanoparticles by fruit extract of Ziziphus spina-christi (L.) Willd: Application for adsorption of triphenylmethane dye and antibacterial assay. J. Mol. Liq. 2018, 255, 541–549. [Google Scholar] [CrossRef]
- Rajesh, K.M.; Ajitha, B.; Reddy, Y.A.K.; Suneetha, Y.; Reddy, P.S. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik 2018, 154, 593–600. [Google Scholar] [CrossRef]
- Lin, Z.; Weng, X.; Owens, G.; Chen, Z. Simultaneous removal of Pb (II) and rifampicin from wastewater by iron nanoparticles synthesized by a tea extract. J. Clean. Prod. 2020, 242, 118476. [Google Scholar] [CrossRef]
- Katata-Seru, L.; Moremedi, T.; Aremu, O.S.; Bahadur, I. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: Removal of nitrate from water and antibacterial activity against Escherichia coli. J. Mol. Liq. 2018, 256, 296–304. [Google Scholar] [CrossRef]
- Radini, I.A.; Hasan, N.; Malik, M.A.; Khan, Z. Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J. Photochem. Photobiol. B 2018, 183, 154–163. [Google Scholar] [CrossRef]
- Liang, T.; Qiu, X.; Ye, X.; Liu, Y.; Li, Z.; Tian, B.; Yan, D. Biosynthesis of selenium nanoparticles and their effect on changes in urinary nanocrystallites in calcium oxalate stone formation. 3 Biotech 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Yazhiniprabha, M.; Vaseeharan, B. In vitro and in vivo toxicity assessment of selenium nanoparticles with significant larvicidal and bacteriostatic properties. Mat. Sci. Eng. C 2019, 103, 109763. [Google Scholar] [CrossRef]
- Menon, S.; KS, S.D.; Agarwal, H.; Shanmugam, V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interf. Sci. Commun. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Din, M.I.; Nabi, A.G.; Rani, A.; Aihetasham, A.; Mukhtar, M. Single step green synthesis of stable nickel and nickel oxide nanoparticles from Calotropis gigantea: Catalytic and antimicrobial potentials. Environ. Nanotechnol. Monit. Manag. 2018, 9, 29–36. [Google Scholar] [CrossRef]
- Sudhasree, S.; Shakila Banu, A.; Brindha, P.; Kurian, G.A. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol. Environ. Chem. 2014, 96, 743–754. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Si, S.; Mandal, T.K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study. Chem. Eur. J. 2007, 13, 3160–3168. [Google Scholar] [CrossRef]
- Anjana, P.M.; Bindhu, M.R.; Umadevi, M.; Rakhi, R.B. Antibacterial and electrochemical activities of silver, gold, and palladium nanoparticles dispersed amorphous carbon composites. Appl. Surf. Sci. 2019, 479, 96–104. [Google Scholar] [CrossRef]
- Dhanavel, S.; Manivannan, N.; Mathivanan, N.; Gupta, V.K.; Narayanan, V.; Stephen, A. Preparation and characterization of cross-linked chitosan/palladium nanocomposites for catalytic and antibacterial activity. J. Mol. Liq. 2018, 257, 32–41. [Google Scholar] [CrossRef]
- Fang, G.; Li, W.; Shen, X.; Perez-Aguilar, J.M.; Chong, Y.; Gao, X.; Chai, Z.; Chen, C.; Ge, C.; Zhou, R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Klaus-Joerger, T.; Joerger, R.; Olsson, E.; Granqvist, C.G. Bacteria as workers in the living factory: Metal-accumulating bacteria and their potential for materials science. Trends Biotechnol. 2001, 19, 15–20. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Alsamhary, K.I. Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Thomas, R.; Janardhanan, A.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz. J. Microbiol. 2014, 45, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monowar, T.; Rahman, M.; Bhore, S.J.; Raju, G.; Sathasivam, K.V. Silver nanoparticles synthesized by using the endophytic bacterium Pantoea ananatis are promising antimicrobial agents against multidrug resistant bacteria. Molecules 2018, 23, 3220. [Google Scholar] [CrossRef] [Green Version]
- Wypij, M.; Golinska, P.; Dahm, H.; Rai, M. Actinobacterial-mediated synthesis of silver nanoparticles and their activity against pathogenic bacteria. IET Nanobiotechnol. 2016, 11, 336–342. [Google Scholar] [CrossRef]
- Husseiny, M.I.; Abd El-Aziz, M.; Badr, Y.; Mahmoud, M.A. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Guo, Z.; Gu, N. Biological synthesis of gold nanowires using extract of Rhodopseudomonas capsulata. Biotechnol. Progr. 2008, 24, 476–480. [Google Scholar] [CrossRef]
- Du, L.; Jiang, H.; Liu, X.; Wang, E. Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem. Commun. 2007, 9, 1165–1170. [Google Scholar] [CrossRef]
- Srinath, B.S.; Namratha, K.; Byrappa, K. Eco-friendly synthesis of gold nanoparticles by Bacillus subtilis and their environmental applications. Adv. Sci. Lett. 2018, 24, 5942–5946. [Google Scholar] [CrossRef]
- Camas, M.; Camas, A.S.; Kyeremeh, K. Extracellular synthesis and characterization of gold nanoparticles using Mycobacterium sp. BRS2A-AR2 isolated from the aerial roots of the Ghanaian mangrove plant, Rhizophora racemosa. Indian J. Microbiol. 2018, 58, 214–221. [Google Scholar] [CrossRef]
- Ahmed, E.; Kalathil, S.; Shi, L.; Alharbi, O.; Wang, P. Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J. Saudi Chem. Soc. 2018, 22, 919–929. [Google Scholar] [CrossRef]
- Tuo, Y.; Liu, G.; Dong, B.; Yu, H.; Zhou, J.; Wang, J.; Jin, R. Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol. Environ. Sci. Pollut. Res. 2017, 24, 5249–5258. [Google Scholar] [CrossRef] [PubMed]
- Eramabadi, P.; Masoudi, M.; Makhdoumi, A.; Mashreghi, M. Microbial cell lysate supernatant (CLS) alteration impact on platinum nanoparticles fabrication, characterization, antioxidant and antibacterial activity. Mat. Sci. Eng. C 2020, 117, 111292. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; He, Z. Bioelectrochemical deposition of palladium nanoparticles as catalysts by Shewanella oneidensis MR-1 towards enhanced hydrogen production in microbial electrolysis cells. Electrochim. Acta 2019, 318, 794–800. [Google Scholar] [CrossRef]
- Che, L.; Dong, Y.; Wu, M.; Zhao, Y.; Liu, L.; Zhou, H. Characterization of selenite reduction by Lysinibacillus sp. ZYM-1 and photocatalytic performance of biogenic selenium nanospheres. ACS Sust. Chem. Eng. 2017, 5, 2535–2543. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surfaces B 2010, 80, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Khatoon, N.; Khan, M.A.; Husain, S.A.; Saravanan, M.; Sardar, M. Synthesis of selenium nanoparticles using probiotic bacteria Lactobacillus acidophilus and their enhanced antimicrobial activity against resistant bacteria. J. Clust. Sci. 2020, 31, 1003–1011. [Google Scholar] [CrossRef]
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.Z.M.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of silver nanoparticles from oropharyngeal Candida glabrata isolates and their antimicrobial activity against clinical strains of bacteria and fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobashigawa, J.M.; Robles, C.A.; Ricci, M.L.M.; Carmarán, C.C. Influence of strong bases on the synthesis of silver nanoparticles (AgNPs) using the ligninolytic fungi Trametes trogii. Saudi J. Biol. Sci. 2019, 26, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 2018, 28, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.A.; Hamzah, H.; Maaroof, M. Analyzing formation of silver nanoparticles from the filamentous fungus Fusarium oxysporum and their antimicrobial activity. Turk. J. Biol. 2018, 42, 54–62. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Alkersh, B.M.; Sabry, S.A.; Ghozlan, H.A. Biosynthesis of silver nanoparticles by Aspergillus terreus: Characterization, optimization, and biological activities. Front. Bioeng. Biotechnol. 2021, 9, 633468. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Nayak, D.; Biswas, K.; Singdevsachan, S.K.; Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Yadav, D.; Mohanta, T.K. Silver nanoparticles synthesized using wild mushroom show potential antimicrobial activities against food borne pathogens. Molecules 2018, 23, 655. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, J.J.A.; Guerrero, D.J.P.; Sáez, R.G.T.; Ishida, K.; Fonseca, B.B.; Rozental, S.; López, C.C.O. Green synthesis of silver nanoparticles using maltose and cysteine and their effect on cell wall envelope shapes and microbial growth of Candida spp. J. Nanosci. Nanotechnol. 2017, 17, 1729–1739. [Google Scholar] [CrossRef] [Green Version]
- Joshi, C.G.; Danagoudar, A.; Poyya, J.; Kudva, A.K.; Dhananjaya, B.L. Biogenic synthesis of gold nanoparticles by marine endophytic fungus—Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 2017, 63, 137–144. [Google Scholar]
- Tripathi, R.M.; Shrivastav, B.R.; Shrivastav, A. Antibacterial and catalytic activity of biogenic gold nanoparticles synthesised by Trichoderma harzianum. IET Nanobiotechnol. 2018, 12, 509–513. [Google Scholar] [CrossRef]
- El Domany, E.B.; Essam, T.M.; Ahmed, A.E.; Farghali, A.A. Biosynthesis physico-chemical optimization of gold nanoparticles as anti-cancer and synergetic antimicrobial activity using Pleurotus ostreatus fungus. J. Appl. Pharm. Sci. 2018, 8, 119–128. [Google Scholar]
- Shen, W.; Qu, Y.; Pei, X.; Li, S.; You, S.; Wang, J.; Zhang, Z.; Zhou, J. Catalytic reduction of 4-nitrophenol using gold nanoparticles biosynthesized by cell-free extracts of Aspergillus sp. WL-Au. J. Hazard. Mater. 2017, 321, 299–306. [Google Scholar] [CrossRef]
- Kitching, M.; Choudhary, P.; Inguva, S.; Guo, Y.; Ramani, M.; Das, S.K.; Marsili, E. Fungal surface protein mediated one-pot synthesis of stable and hemocompatible gold nanoparticles. Enzym. Microb. Technol. 2016, 95, 76–84. [Google Scholar] [CrossRef]
- Subramaniyan, S.A.; Sheet, S.; Vinothkannan, M.; Yoo, D.J.; Lee, Y.S.; Belal, S.A.; Shim, K.S. One-pot facile synthesis of Pt nanoparticles using cultural filtrate of microgravity simulated grown P. chrysogenum and their activity on bacteria and cancer cells. J. Nanosci. Nanotechnol. 2018, 18, 3110–3125. [Google Scholar] [CrossRef] [PubMed]
- Riddin, T.L.; Gericke, M.; Whiteley, C.G. Analysis of the inter-and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 2006, 17, 3482. [Google Scholar]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Soliman, H.; Elsayed, A.; Dyaa, A. Antimicrobial activity of silver nanoparticles biosynthesised by Rhodotorula sp. strain ATL72. Egypt. J. Basic Appl. Sci. 2018, 5, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Korbekandi, H.; Mohseni, S.; Mardani Jouneghani, R.; Pourhossein, M.; Iravani, S. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif. Cell Nanomed. Biotechnol. 2016, 44, 235–239. [Google Scholar] [CrossRef]
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process Biochem. 2016, 51, 1306–1313. [Google Scholar] [CrossRef]
- Cunha, F.A.; da CSO Cunha, M.; da Frota, S.M.; Mallmann, E.J.; Freire, T.M.; Costa, L.S.; Paula, A.J.; Menezes, E.A.; Fechine, P.B. Biogenic synthesis of multifunctional silver nanoparticles from Rhodotorula glutinis and Rhodotorula mucilaginosa: Antifungal, catalytic and cytotoxicity activities. World J. Microbiol. Biotechnol. 2018, 34, 1–15. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Z.; Lu, X.; He, F.; Zhu, X.; Ma, Y.; He, R.; Gao, F.; Ni, W.; Yi, Y. Controllable biosynthesis and properties of gold nanoplates using yeast extract. Nano Micro Lett. 2017, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; You, S.; Zhang, X.; Pei, X.; Shen, W.; Li, Z.; Li, S.; Zhang, Z. Biosynthesis of gold nanoparticles using cell-free extracts of Magnusiomyces ingens LH-F1 for nitrophenols reduction. Bioprocess Biosyst. Eng. 2018, 41, 359–367. [Google Scholar] [CrossRef]
- Sriramulu, M.; Sumathi, S. Biosynthesis of palladium nanoparticles using Saccharomyces cerevisiae extract and its photocatalytic degradation behaviour. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025018. [Google Scholar] [CrossRef]
- Lian, S.; Diko, C.S.; Yan, Y.; Li, Z.; Zhang, H.; Ma, Q.; Qu, Y. Characterization of biogenic selenium nanoparticles derived from cell-free extracts of a novel yeast Magnusiomyces ingens. 3 Biotech 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Vashisth, P.; Pruthi, V.; Chopade, B.A. Kinetics of synthesis of gold nanoparticles by Acinetobacter sp. SW30 isolated from Environment. Indian J. Microbiol. 2016, 56, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.B.; Sun, D.; Huang, J.; Odoom-Wubah, T.; Li, Q. State of arts on the bio-synthesis of noble metal nanoparticles and their biological application. Chin. J. Chem. Eng. 2020, 30, 272–290. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Sabri, M.A.; Umer, A.; Awan, G.H.; Hassan, M.F.; Hasnain, A. Selection of suitable biological method for the synthesis of silver nanoparticles. Nanomater. Nanotechnol. 2016, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.H. A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett. 2014, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Yun, Y.S. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour. Technol. 2010, 101, 7958–7965. [Google Scholar] [CrossRef]
- Armendariz, V.; Herrera, I.; Jose-yacaman, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanoparticle Res. 2004, 6, 377–382. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Progr. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Punia, P.; Bharti, M.K.; Chalia, S.; Dhar, R.; Ravelo, B.; Thakur, P.; Thakur, A. Recent advances in synthesis, characterization, and applications of nanoparticles for contaminated water treatment—A review. Ceram. Int. 2021, 47, 1526–1550. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Nadiminti, P.P.; Dong, Y.D.; Sayer, C.; Hay, P.; Rookes, J.E.; Boyd, B.J.; Cahill, D.M. Nanostructured liquid crystalline particles as an alternative delivery vehicle for plant agrochemicals. ACS Appl. Mater. Interfaces 2013, 5, 1818–1826. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Lee, J.; Jayaprakasha, G.K.; Patil, B.S. Seed priming with iron oxide nanoparticles modulate antioxidant potential and defense linked hormones in watermelon seedlings. ACS Sust. Chem. Eng. 2019, 7, 5142. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, C.; Wen, J.; Wu, G.; Tao, M. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–171. [Google Scholar]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Li, J.; Chang, P.R.; Huang, J.; Wang, Y.; Yuan, H.; Ren, H. Physiological effects of magnetic iron oxide nanoparticles towards watermelon. J. Nanosci. Nanotechnol. 2013, 13, 5561–5567. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum) seeds Mill. Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Mazaheri-Tirani, M.; Dayani, S. In vitro effect of zinc oxide nanoparticles on Nicotiana tabacum callus compared to ZnO micro particles and zinc sulfate (ZnSO4). Plant Cell Tissue Organ Cult. 2020, 140, 279–289. [Google Scholar] [CrossRef]
- Noori, A.; Donnelly, T.; Colbert, J.; Cai, W.; Newman, L.A.; White, J.C. Exposure of tomato (Lycopersicon esculentum) to silver nanoparticles and silver nitrate: Physiological and molecular response. Int. J. Phytoremediation 2020, 22, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Rodrigo, M.A.M.; Moulick, A.; Heger, Z.; Kopel, P.; Zitka, O.; Adam, V.; Lukatkin, A.S.; Duarte, A.C.; Pereira, E. Transport phenomena of nanoparticles in plants and animals/humans. Environ. Res. 2016, 151, 233–243. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Green-synthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L.). ACS Sust. Chem. Eng. 2019, 7, 14580–14590. [Google Scholar] [CrossRef]
- Sehnal, K.; Hosnedlova, B.; Docekalova, M.; Stankova, M.; Uhlirova, D.; Tothova, Z.; Kepinska, M.; Milnerowicz, H.; Fernandez, C.; Ruttkay-Nedecky, B.; et al. An assessment of the effect of green synthesized silver nanoparticles using sage leaves (Salvia officinalis L.) on germinated plants of maize (Zea mays L.). Nanomaterials 2019, 9, 1550. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Rafique, S.; Zafar, A.; Loomba, S.; Khan, R.; Hassan, S.G.; Khan, M.W.; Zahra, S.; Zia, M.; Mustafa, G.; et al. Physiological and anti-oxidative response of biologically and chemically synthesized iron oxide: Zea mays a case study. Heliyon 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-De-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- Rameshaiah, G.N.; Pallavi, J.; Shabnam, S. Nano fertilizers and nano sensors—An attempt for developing smart agriculture. Int. J. Eng. Res. Gen. Sci. 2015, 3, 314–320. [Google Scholar]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Mastronardi, E.; Tsae, P.; Zhang, X.; Monreal, C.; DeRosa, M.C. Strategic role of nano-technology in fertilizers: Potential and limitations. In Nanotechnologies in Food and Agriculture; Springer: Berlin/Heidelberg, Germany, 2015; pp. 25–67. [Google Scholar]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Patidar, V.; Jain, P. Green synthesis of TiO2 nanoparticle using Moringa oleifera leaf extract. Int. Res. J. Eng. Technol. 2017, 4, 470–473. [Google Scholar]
- Abisharani, J.M.; Devikala, S.; Kumar, R.D.; Arthanareeswari, M.; Kamaraj, P. Green synthesis of TiO2 nanoparticles using Cucurbita pepo seeds extract. Mater. Today Proc. 2019, 14, 302–307. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agro. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef] [Green Version]
- Delfani, M.; Baradarn Firouzabadi, M.; Farrokhi, N.; Makarian, H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 2014, 45, 530–540. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef] [PubMed]
- Jampílek, J.; Králová, K. Nanopesticides: Preparation, targeting, and controlled release. In New Pesticides and Soil Sensors; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 81–127. [Google Scholar]
- Sasson, Y.; Levy-Ruso, G.; Toledano, O.; Ishaaya, I. Nanosuspensions: Emerging novel agrochemical formulations. In Insecticides Design Using Advanced Technologies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–39. [Google Scholar]
- Paret, M.L.; Vallad, G.E.; Averett, D.R.; Jones, J.B.; Olson, S.M. Photocatalysis: Effect of light-activated nanoscale formulations of TiO2 on Xanthomonas perforans and control of bacterial spot of tomato. Phytopathology 2013, 103, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Pearce, R.C.; Melechko, A.V.; Hensley, D.K.; Simpson, M.L.; Rack, P.D. Pulsed laser dewetting of nickel catalyst for carbon nanofiber growth. Nanotechnology 2008, 19, 235604. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H. Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agricul. Food. Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Tech. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- China Research and Intelligence. Global Nanotechnology for Food Packaging Market to Grow at 13.6% CAGR, Which Is Anticipated to Reach USD 44.8 billion by 2030; China Research and Intelligence Co. Ltd.: Shanghai, China, 2021. [Google Scholar]
- Assefa, Z.; Admassu, S. Development and characterization of antimicrobial packaging films. J. Food Process Technol. 2013, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kalita, D.; Baruah, S. The impact of nanotechnology on food. In Nanomaterials Applications for Environmental Matrices; Do Nascimento, R., Ferreira, O.P., De Paula, A.J., Neto, V.D.O.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–379. [Google Scholar]
- Krishna, R.; Campina, J.M.; Fernandes, P.M.V.; Ventura, J.; Titus, E.; Silva, A.F. Reduced graphene oxide-nickel nanoparticles/biopolymer composite films for sub-millimolar detection of glucose. Analyst 2016, 141, 4151–4161. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Shen, Y.; Yang, M.; Li, X.; Han, X.; Jiang, X.; Zhao, B. SERS strategy based on the modified Au nanoparticles for highly sensitive detection of bisphenol A residues in milk. Talanta 2018, 179, 37–42. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kanmani, P.; Rhim, J.W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, J.M. Nanotechnology in Food Products: Workshop Summary; Institute of Medicine (US) Food Forum; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501. [Google Scholar] [CrossRef] [Green Version]
- Graveland-Bikker, J.F.; De Kruif, C.G. Unique milk protein based nanotubes: Food and nanotechnology meet. Trends Food Sci. Technol. 2006, 17, 196–203. [Google Scholar] [CrossRef]
- Oberdörster, G.; Stone, V.; Donaldson, K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology 2007, 1, 2–25. [Google Scholar] [CrossRef]
- Fernandez, A.; Cava, D.; Ocio, M.J.; Lagaron, M. Perspectives for biocatalysts in food packaging. Trends Food Sci. Technol. 2008, 19, 198–206. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, S.; Ojha, N.; Shrivastava, A.; Barla, A.; Rai, V.; Bose, S. Facets of nanotechnology as seen in food processing, packaging, and preservation industry. BioMed Res. Int. 2015, 2015, 365672. [Google Scholar] [CrossRef] [Green Version]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Goetz, N.V. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Tommas, D.; Gianfranco, D.D.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Motlagh, N.V.; Mosavian, M.T.H.; Mortazavi, S.A. Effect of polyethylene packaging modified with silver particles on the microbial, sensory and appearance of dried barberry. Packag. Technol. Sci. 2012, 26, 39–49. [Google Scholar] [CrossRef]
- Yang, F.M.; Li, H.M.; Li, F.; Xin, Z.H.; Zhao, L.Y.; Zheng, Y.H.; Hu, Q.H. Effect of nano-packing on preservation quality of fresh strawberry (Fragaria ananassa Duch. Cv Fengxiang) during storage at 4 °C. J. Food Chem. 2010, 75, C236–C240. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Abad, A.; Lagaron, J.M.; Ocio, M.J. Development and characterization of silver-based antimicrobial ethylene-vinyl alcohol copolymer (EVOH) films for food-packaging applications. J. Agric. Food Chem. 2012, 60, 5350–5359. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, S.S.; Vadood, R.; Nourdahr, R. Study on the antimicrobial effect of nanosilver tray packaging of minced beef at refrigerator temperature. Glob. Vet. 2012, 9, 284–289. [Google Scholar]
- Metak, A.M.; Ajaal, T.T. Investigation on polymer based nanosilver as food packaging materials. Int. J. Biol. Food Vet. Agric. Eng. 2013, 7, 772–777. [Google Scholar]
- Metak, A.M. Effects of nanocomposites based nano-silver and nano-titanium dioxideon food packaging materials. Int. J. Appl. Sci. Technol. 2015, 5, 26–40. [Google Scholar]
- Akbari, Z.; Ghomashchi, T.; Aroujalian, A. Potential of nanotechnology for food packaging industry. In Proceedings of the “Nano Micro Technologies in the Food Health Food Industries” Conference organised by Institute of Nanotechnology, Amsterdam, The Netherland, 25–26 October 2006. [Google Scholar]
- Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Morsy, M.K. Stability of antimicrobial activity of pullulan edible films incorporated with nanoparticles and essential oils and their impact on turkey deli meat quality. J. Food Dairy Sci. 2013, 4, 557–573. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C. Toxicology of nanoparticles. Adv. Drug Deliver. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, R. Nanomedicine—The future of cancer treatment: A Review. J. Cancer Prev. Curr. Res. 2017, 8, 00265. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M.; et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B 2017, 167, 282–289. [Google Scholar] [CrossRef]
- Elangovan, K.; Elumalai, D.; Anupriya, S.; Shenbhagaraman, R.; Kaleena, P.; Murugesan, K. Phyto mediated biogenic synthesis of silver nanoparticles using leaf extract of Andrographis echioides and its bio-efficacy on anticancer and antibacterial activities. J. Photochem. Photobiol. B 2015, 151, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, S.; Velmurugan, B.; Bhuvaneswari, V.; Nagini, S. Effects of aqueous extracts of garlic (Allium sativum) and neem (Azadirachta indica) leaf on hepatic and blood oxidant-antioxidant status during experimental gastric carcinogenesis. J. Med. Food 2004, 7, 334–339. [Google Scholar] [CrossRef]
- Adewale, O.; Davids, H.; Cairncross, L.; Roux, S. Toxicological behavior of gold nanoparticles on various models: Influence of physicochemical properties and other factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef]
- Rao, P.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S. Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents. Oxid. Med. Cell. Longev. 2016, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Farshchi, H.; Azizi, M.; Jaafari, M.; Nemati, S.; Fotovat, A. Green synthesis of iron nanoparticles by Rosemary extract and cytotoxicity effect evaluation on cancer cell lines. Biocatal. Agric. Biotechnol. 2018, 16, 54–62. [Google Scholar] [CrossRef]
- Kostyukova, D.; Chung, Y. Synthesis of iron oxide nanoparticles using isobutanol. J. Nanomater. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Peng, X.H.; Qian, X.; Mao, H.; Wang, A.Y.; Chen, Z.G.; Nie, S.; Shin, D.M. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int. J. Nanomed. 2008, 3, 311–321. [Google Scholar]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.K.; Chattopadhyaya, M.C. Nanomaterials for Wastewater Remediation; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Sadegh, H.; Ali, G.A.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dil, E.A.; Ghaedi, M.; Asfaram, A. The performance of nanorods material as adsorbent for removal of azo dyes and heavy metal ions: Application of ultrasound wave, optimization and modeling. Ultrason. Sonochem. 2017, 34, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- El-Sayed, M.E. Nanoadsorbents for water and wastewater remediation. Sci. Total Environ. 2020, 739, 139903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, B.; Theng, B.K.; Wu, P.; Liu, F.; Wang, S.; Lee, X.; Chen, M.; Li, L.; Zhang, X. Formation and mechanisms of nano-metal oxide-biochar composites for pollutant removal: A review. Sci. Total Environ. 2021, 767, 145305. [Google Scholar] [CrossRef]
- Das, C.; Sen, S.; Singh, T.; Ghosh, T.; Paul, S.S.; Kim, T.W.; Jeon, S.; Maiti, D.K.; Im, J.; Biswas, G. Green synthesis, characterization and application of natural product coated magnetite nanoparticles for wastewater treatment. Nanomaterials 2020, 10, 1615. [Google Scholar] [CrossRef]
- Al-Qahtani, K.M. Cadmium removal from aqueous solution by green synthesis zero valent silver nanoparticles with Benjamina leaves extract. Egypt. J. Aquat. Res. 2017, 43, 269–274. [Google Scholar] [CrossRef]
- Ehrampoush, M.H.; Miria, M.; Salmani, M.H.; Mahvi, A.H. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J. Environ. Health Sci. 2015, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primo, J.D.O.; Bittencourt, C.; Acosta, S.; Sierra-Castillo, A.; Colomer, J.F.; Jaerger, S.; Teixeira, V.C.; Anaissi, F.J. Synthesis of Zinc Oxide nanoparticles by ecofriendly routes: Adsorbent for copper removal from wastewater. Front. Chem. 2020, 8, 571790. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Hu, Y.; Wang, R.; Chen, M.; Wu, J.; Wang, Y.; Kang, S.; Sun, Y.; Zhu, M. Behavior, remediation effect and toxicity of nanomaterials in water environments. Environ. Res. 2019, 174, 54–60. [Google Scholar] [CrossRef]
- Wang, P.; Wang, F.; Jiang, H.; Zhang, Y.; Zhao, M.; Xiong, R.; Ma, J. Strong improvement of nanofiltration performance on micropollutant removal and reduction of membrane fouling by hydrolyzed-aluminum nanoparticles. Water Res. 2020, 175, 115649. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; D’asti, V.; Piaggio, P. Preparation and properties of novel organic-inorganic porous membranes. Sep. Purif. Technol. 2001, 22, 269–275. [Google Scholar] [CrossRef]
- Fujiwara, M.; Imura, T. Photo induced membrane separation for water purification and desalination using azobenzene modified anodized alumina membranes. ACS Nano 2015, 9, 5705–5712. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wong, K.; Wan, W. Optimization of Al2O3/PES membranes for wastewater filtration. Sep. Purif. Technol. 2010, 73, 294–301. [Google Scholar] [CrossRef]
- Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard. Mater. 2016, 309, 53–64. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Mauter, M.S.; Wang, Y.; Okemgbo, K.C.; Osuji, C.O.; Giannelis, E.P.; Elimelech, M. Antifouling ultrafiltration membranes via post-fabrication grafting of biocidal nanomaterials. ACS Appl. Mater. Inter. 2011, 3, 2861–2868. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Patanjali, P.; Singh, R.; Kumar, A.; Chaudhary, P. Nanotechnology for water treatment: A green approach. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 485–512. [Google Scholar]
- Anjum, M.; Al-Makishah, N.H.; Barakat, M.A. Wastewater sludge stabilization using pre-treatment methods. Process Saf. Environ. 2016, 102, 615–632. [Google Scholar] [CrossRef]
- Hemmati, S.; Mehrazin, L.; Ghorban, H.; Garakani, S.H.; Mobaraki, T.H.; Mohammadi, P.; Veisi, H. Green synthesis of Pd nanoparticles supported on reduced graphene oxide, using the extract of Rosa canina fruit, and their use as recyclable and heterogeneous nanocatalysts for the degradation of dye pollutants in water. RSC Adv. 2018, 8, 21020–21028. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Smita, K.; Cumbal, L. Phytosynthesis of gold nanoparticles using Andean Ajı′ (Capsicum baccatum L.). Cogent Chem. 2015, 1, 1120982. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. One pot synthesis and characterization of gold nanocatalyst using Sacha inchi (Plukenetia volubilis) oil: Green approach. J. Photochem. Photobiol. B 2016, 158, 55–60. [Google Scholar] [CrossRef]
- Sumi, M.B.; Devadiga, A.; Shetty, K.V.; Saidutta, M.B. Solar photocatalytically active, engineered silver nanoparticle synthesis using aqueous extract of mesocarp of Cocos nucifera (Red Spicata Dwarf). J. Exp. Nanosci. 2017, 12, 14–32. [Google Scholar] [CrossRef] [Green Version]
- Tahir, K.; Nazir, S.; Li, B.; Khan, A.U.; Khan, Z.U.H.; Ahmad, A.; Khan, F.U. An efficient photo catalytic activity of green synthesized silver nanoparticles using Salvadora persica stem extract. Sep. Purif. Technol. 2015, 150, 316–324. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Prabhakar Vattikuti, S.V.; Devarayapalli, K.C.; Yoo, K.; Shim, J.; Sreekanth, T.V.M. Green synthesis: Photocatalytic degradation of textile dyes using metal and metal oxide nanoparticles-latest trends and advancements. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2617–2723. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. Nanoantibiotics: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Hughes, J.M. The challenges of emerging infectious diseases: Development and spread of multiply-resistant bacterial pathogens. JAMA 1996, 275, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Li, J.P.; Qian, K.; Xu, W.P.; Lu, Y.; Huang, W.X.; Yu, S.H. Large scale photochemical synthesis of M@TiO2 nanocomposites (M = Ag, Pd, Au, Pt) and their optical properties, CO oxidation performance, and antibacterial effect. Nano Res. 2010, 3, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Rajendra, R.; Balakumar, C.; Ahammed, H.A.M.; Jayakumar, S.; Vaideki, K.; Rajesh, E. Use of zinc oxide nano particles for production of antimicrobial textiles. Int. J. Eng. Sci. Technol. 2010, 2, 202–208. [Google Scholar] [CrossRef]
- Kuang, Y.; He, X.; Zhang, Z.; Li, Y.; Zhang, H.; Ma, Y.; Wu, Z.; Chai, Z. Comparison study on the antibacterial activity of nano- or bulk-cerium oxide. J. Nanosci. Nanotechnol. 2011, 11, 4103–4108. [Google Scholar] [CrossRef]
- Li, S.-T.; Qiao, X.-L.; Chen, J.-G.; Wu, C.-L.; Mei, B. The investigation of antibacterial characteristics of magnesium oxide and it’s nano-composite materials. J. Funct. Mater. 2005, 11, 1651–1654. [Google Scholar]
- Srivastava, M.; Singh, S.; Self, W.T. Exposure to silver nanoparticles inhibits selenoprotein synthesis and the activity of thioredoxin reductase. Environ. Health Perspect. 2012, 120, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Patel, P.; Jaiswal, S.; Prabhune, A.A.; Ramana, C.V.; Prasad, B.L.V. A direct method for the preparation of glycolipidmetal nanoparticle conjugates: Sophorolipids as reducing and capping agents for the synthesis of water re-dispersible silver nanoparticles and their antibacterial activity. New J. Chem. 2009, 33, 646–652. [Google Scholar] [CrossRef]
- Masoumbaigi, H.; Rezaee, A.; Hosseini, H.; Hashemi, S. Water disinfection by zinc oxide nanoparticle prepared with solution combustion method. Desalin. Water Treat. 2015, 56, 2376–2381. [Google Scholar] [CrossRef]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Adibkia, K.; Alaei-Beirami, M.; Barzegar-Jalali, M.; Mohammadi, G.; Ardestani, M.S. Evaluation and optimization of factors affecting novel diclofenac sodium-eudragit RS100 nanoparticles. Afr. J. Pharm. Pharmacol. 2012, 6, 941–947. [Google Scholar]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [Green Version]
- Adibkia, K.; Barzegar-Jalali, M.; Nokhodchi, A.; Siahi Shadbad, M.; Omidi, Y.; Javadzadeh, Y.; Mohammadi, G. A review on the methods of preparation of pharmaceutical nanoparticles. J. Pharm. Sci. 2010, 15, 303–314. [Google Scholar]
- Hoseinzadeh, E.; Alikhani, M.-Y.; Samarghandi, M.-R.; Shirzad-Siboni, M. Antimicrobial potential of synthesized zinc oxide nanoparticles against gram positive and gram negative bacteria. Desalin. Water Treat. 2014, 52, 4969–4976. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Firouzabadi, F.B. Reduction of listeria monocytogenes and Bacillus cereus in milk by zinc oxide nanoparticles. Iran J. Pathol. 2015, 10, 97–104. [Google Scholar]
- Wongyai, K.; Wintachai, P.; Maungchang, R.; Rattanakit, P. Exploration of the antimicrobial and catalytic properties of gold nanoparticles greenly synthesized by Cryptolepis buchanani Roem. and Schult extract. J. Nanomater. 2020, 2020, 1320274. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.A.R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected Gram-negative food borne pathogens, Front. Microbiol. 2018, 9, 1555. [Google Scholar]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bera, R.K.; Mandal, S.M.; Raj, C.R. Antimicrobial activity of fluorescent Ag nanoparticles. Lett. Appl. Microbiol. 2014, 58, 520–526. [Google Scholar] [CrossRef]
- Song, Y.; Chen, L. Effect of net surface charge on physical properties of the cellulose nanoparticles and their efficacy for oral protein delivery. Carbohyd. Polym. 2015, 121, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, J.S.; Maynard, A.D.; Howard, P.C.; James, J.T.; Lam, C.-W.; Warheit, D.B.; Santamaria, A.B. Research strategies for safety evaluation of nanomaterials, part IV: Risk assessment of nanoparticles. Toxicol. Sci. 2006, 89, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef] [Green Version]
- Zinjarde, S.S. Bio-inspired nanomaterials and their applications as antimicrobial agents. Chron. Young Sci. 2012, 3, 1–74. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles—A material of the future? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Giorgetti, L. Effects of nanoparticles in plants: Phytotoxicity and genotoxicity assessment. Nanomater. Plants Algae Microorg. 2019, 2, 65–87. [Google Scholar]
- Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schäffler, M.; Tian, F.; Schmid, G.; Oberdörster, G.; Kreyling, W.G. Size dependent translocation and fetal accumulation of gold nanoparticles from maternal blood in the rat. Part. Fibre Toxicol. 2014, 11, 1–12. [Google Scholar] [CrossRef]
- Gao, G.; Ze, Y.; Li, B.; Zhao, X.; Zhang, T.; Sheng, L.; Hu, R.; Gui, S.; Sang, X.; Sun, Q.; et al. Ovarian dysfunction and gene–expressed characteristics of female mice caused by long–term exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2012, 243, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.R.; Kundu, C.N. Promising opportunities and potential risk of nanoparticle on the society. IET Nanobiotechnol. 2020, 14, 253–260. [Google Scholar] [CrossRef]
- Wang, W.N.; Tarafdar, J.C.; Biswas, P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanoparticle. Res. 2013, 15, 1417. [Google Scholar] [CrossRef]
- Tripathi, A.; Liu, S.; Singh, P.K.; Kumar, N.; Pandey, A.C.; Tripathi, D.K.; Chauhan, D.K.; Sahi, S. Differential phytotoxic responses of silver nitrate (AgNO3) and silver nanoparticle (AgNps) in Cucumis sativus L. Plant Gene 2017, 11, 255–264. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Chen, M.; Liu, X.; Xiong, Y.; Wang, Y.; Feng, T.; Kang, S.; Wang, X. Recent advances in the biotoxicity of metal oxide nanoparticles: Impacts on plants, animals and microorganisms. Chemosphere 2019, 237, 124403. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Patel, M.K.; Shah, A.; Kumar, V.; Gantait, S. Engineered nanomaterials for plant growth and development: A perspective analysis. Sci. Total Environ. 2018, 630, 1413–1435. [Google Scholar] [CrossRef] [PubMed]
- Barbafieri, M.; Giorgetti, L. Contaminant bioavailability in soil and phytotoxicity/genotoxicity tests in Vicia faba L.: A case study of boron contamination. Environ. Sci. Pollut. Res. Int. 2016, 23, 24327–24336. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Subba Rao, A. Impact of SiO2 and Mo nanoparticles on seed germination of rice (Oryza sativa L.). Int. J. Agric. Food Sci. Technol. 2013, 4, 809–816. [Google Scholar]
- Qados, A.M.S.A. Mechanism of nanosilicon–mediated alleviation of salinity stress in faba bean (Vicia faba L.). Plants Am. J. Exp. Agric. 2015, 7, 78–95. [Google Scholar] [CrossRef]
- Almutairi, Z.M. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (“Solanum lycopersicum” L.) seedlings under salt stress. Plant Omics 2016, 9, 106–114. [Google Scholar]
- Zmeeva, O.; Daibova, E.; Proskurina, L.; Petrova, L.V.; Kolomiets, N.E.; Svetlichny, V.A.; Lapin, I.N.; Karakchieva, N.I. Effects of silicon dioxide nanoparticles on biological and physiological characteristics of Medicago sativa L. nothosubsp. varia (Martyn) in natural agroclimaticc of the subtaiga zone in western Siberia. Bionanoscience 2017, 7, 672–679. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Schoenfisch, M.H. Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ. Sci. Technol. 2012, 46, 10247–10254. [Google Scholar] [PubMed]
- Karimi, J.; Mohsenzadeh, S. Effects of silicon oxide nanoparticles on growth and physiology of wheat seedlings. Russ. J. Plant Physiol. 2016, 63, 119–123. [Google Scholar] [CrossRef]
- Silva, G.H.; Monteiro, R.T.R. Toxicity assessment of silica nanoparticles on Allium cepa. Ecotoxicol. Environ. Contam. 2017, 12, 25–31. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanoparticle Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Cremonini, R.; Bottega, S.; Spano, C. Impact of TiO2 nanoparticles on Vicia narbonensis L.: Potential toxicity effects. Protoplasma 2014, 251, 1471–1479. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Becarelli, S.; Siracusa, G.; Lorenzi, R.; Di Gregorio, S. Polycyclic aromatic hydrocarbon-contaminated soils: Bioaugmentation of autochthonous bacteria and toxicological assessment of the bioremediation process by means of Vicia faba L. Environ. Sci. Pollut. Res. Int. 2016, 23, 7930–7941. [Google Scholar] [CrossRef]
- Ruffini Castiglione, M.; Giorgetti, L.; Bellani, L.; Muccifora, S.; Bottega, S.; Spano, C. Root responses to different types of TiO2 nanoparticles and bulk counterpart in plant model system Vicia faba L. Environ. Exp. Bot. 2016, 130, 11–21. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2012, 78, 80–85. [Google Scholar] [CrossRef]
- Dash, A.; Singh, A.P.; Chaudhary, B.R.; Singh, S.K.; Dash, D. Effect of silver nanoparticles on growth of eukaryotic green algae. Nanomicro Lett. 2012, 4, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Bakand, S.; Hayes, A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef]
- Oberdörster, G.; Elder, A.; Rinderknecht, A. Nanoparticles and the brain: Cause for concern? J. Nanosci. Nanotechnol. 2009, 9, 4996–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, A.; Tentschert, J.; Jungnickel, H. Toxicity of silver nanoparticles in human macrophages: Uptake, intracellular distribution and cellular responses. J. Phys. Conf. Ser. 2011, 304, 012030. [Google Scholar] [CrossRef] [Green Version]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of titanium dioxide nanoparticles exposure on human health—A review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, M.; Kim, M.; Cho, H.; Lee, J.; Yu, J.; Chung, H.; Choi, S. Factors influencing the cytotoxicity of zinc oxide nanoparticles: Particle size and surface charge. J. Phys. Conf. Ser. 2011, 304, 012044. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, S.; Liu, Y.; Xie, H.; Luo, B.; Wang, J. In vitro inhibition of tumor growth by low-dose iron oxide nanoparticles activating macrophages. J. Biomater. Appl. 2019, 33, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Anna, M.; Oksana, V.; Jana, K. Effect of chemically and biologically synthesized Ag nanoparticles on the algae growth inhibition. AIP Conf. Proc. 2017, 1918, 020008. [Google Scholar]







| Nanoparticles | Plant Species | Experimental Conditions | Shape and Size | References |
|---|---|---|---|---|
| Silver (Ag) | Acalypha indica Linn | Temperature: 27 °C; pH: 7.0; duration: 30 min | Spherical; 20–30 nm | [26] |
| Chenopodium album leaf | Temperature: 20–100 °C; pH: 2.0–10.0; duration: 15 min | Spherical; 10–30 nm | [27] | |
| Hibiscus rosa sinensis leaf | pH: 7.2–8.5 | Spherical; 13 nm | [28] | |
| Calendula officinalis seed | Temperature: 30 and 60 °C; pH: 3.0–9.0 | Spherical; 7.5 nm | [29] | |
| Allophylus cobbe leaf | Temperature: 60 °C; pH: 8.0; duration: 6 h | Spherical; 2–10 nm | [30] | |
| Cissusquadrangularis leaf | Duration: 60 min | Spherical and cuboidal | [31] | |
| Piper nigrum, Ziziphus Spina—Christi and Eucalyptus globulus leaves | Temperature: ambient; duration: 1 h | Spherical; 8−35 nm | [32] | |
| Phyllanthus emblica fruit | Temperature: 65 °C; duration: 2 h | Spherical; 16.29 nm | [33] | |
| Blumea eriantha DC | Temperature: ambient; duration: 2–3 h | Spherical; 50 nm | [34] | |
| Brillantaisia patula, Crossopteryx febrifuga and Senna siamea leaf | Temperature: 70 °C; duration: 24 h | Spherical; 45–110 nm | [35] | |
| Ocimum tenuiflorum leaf | Temperature: ambient; duration: 10 min | Spherical and ovoid; 7–15 nm | [36] | |
| Annona squamosa leaf | Temperature: ambient | Spherical; 20–100 nm | [37] | |
| Aloe leaf | Temperature: ambient; duration: 20 min | Spherical; 20 nm | [38] | |
| Artocarpus heterophyllus Lam. Seed | Temperature: 121 °C; duration: 5 min | Irregular; 3–25 nm | [39] | |
| Trigonella foenum graecum seed | Duration: 5 min | Spherical; 17 nm | [40] | |
| Andrographis paniculata | Temperature: 30–95 °C | Spherical; 13–27 nm | [41] | |
| Podophyllum hexandrum leaf | Temperature: 20–60 °C; pH: 4.5–10.0; duration: 30–150 min | Spherical; 12–40 nm | [42] | |
| Syzygium cumini fruit | Temperature: ambient; pH: 7.0–9.0; duration: 2 h | Spherical; 5–20 nm | [43] | |
| Crassocephalum rubens leaf | Temperature: 50 °C; duration: 20 min | Spherical and hexagonal; 15–25 nm | [44] | |
| Gold (Au) | Cassia fistula stem bark | Temperature: ambient | Rectangular and triangular; 55.2–98.4 nm | [45] |
| Crassocephalum rubens leaf | Temperature: 50 °C; duration: 10 min | Spherical; 10–20 nm | [44] | |
| Simarouba glauca leaf | Duration: 15 min | Spherical and prism; <10 nm | [46] | |
| Hygrophila spinosa | Temperature: 30–100 °C; pH:2.0–12.0; duration: 15–60 min | Spherical, polygonal, rod and triangular; 68 nm | [47] | |
| Croton Caudatus Geisel leaf | Temperature: ambient | Spherical; 20–50 nm | [48] | |
| Moringa oleifera flower | Temperature: ambient; duration: 60 min | Triangular, hexagonal, and spherical; 5 nm | [49] | |
| Illicium verum | Temperature: 25–50 °C; pH: 2.0–10.00; duration: 15 min | Triangular and hexagonal; 20–50 nm | [50] | |
| Terminalia arjuna leaf | Temperature: ambient; duration: 15 min | Spherical; 20–50 nm | [51] | |
| Zingiber officinale | Temperature: 37 and 50 °C; pH: 7.4; duration: 20 min | Spherical; 5–10 nm | [52] | |
| Rosa hybrida petal | Temperature: ambient; duration: 5 min | Spherical, triangular, and hexagonal; 10 nm. | [53] | |
| Terminalia chebula seed | Temperature: ambient; duration: 20 s | Triangular, pentagonal, and spherical; 6–60 nm | [54] | |
| Eucommia ulmoides bark | Temperature: 30–60 °C; pH: 5.0– 13.0; duration: 30 min | Spherical | [55] | |
| Acorus calamus rhizome | Temperature: ambient; pH: 4.0–9.2 | Spherical; 10 nm | [56] | |
| Curcuma pseudomontana root | Temperature: ambient; duration: 30 min | Spherical shape; 20 nm | [57] | |
| Citrus limon, Citrus reticulata and Citrus sinensis | Temperature: ambient; duration: 10 min | Spherical and triangular; 15–80 nm | [58] | |
| Palladium (Pd) | Hippophae rhamnoides Linn leaf | Temperature: 80 °C; duration: 25 min | 2.5–14 nm | [59] |
| Cinnamom zeylanicum bark extract | Temperature: 30 °C; pH: 1.0–11.0; duration: 72 h | Spherical; 15–20 nm | [60] | |
| Banana peel extract | Temperature: 40–100 °C; pH: 2.0–5.0; duration: 3 min | 50 nm | [61] | |
| Cinnamomum camphora leaf | Temperature: ambient; duration: 12 h | Quasi–spherical and irregular; 3.6–9.9 nm | [62] | |
| Catharanthus roseus leaf | Temperature: 60 °C; duration: 2 h | Spherical; 38 nm | [63] | |
| Terminalia chebula fruit | Temperature: ambient; duration: 40 min | - | [64] | |
| Rosmarinus officinalis | Temperature: ambient; duration: 24 h | Semi–spherical; 15–90 nm | [65] | |
| Anogeissus latifolia | Duration: 30 min | Spherical; 2.3–7.5 nm | [66] | |
| Daucus carota leaves | - | Rod; diameter—20 nm, length—38–48 nm | [67] | |
| Camellia sinensis leaves | Temperature: 100 °C; duration: 1 h | Spherical; 5–8 nm | [68] | |
| Platinum (Pt) | Anacardium occidentale leaf | Temperature: ambient; pH: 6.0–8.0 | Irregular rod shaped | [69] |
| Cacumen platycladi | Temperature: 30–90 °C; duration: 25 h | Spherical; 2–2.9 nm | [70] | |
| Asparagus racemosus root | Duration: 5 min | 1.0–6.0 nm | [71] | |
| Diopyros kaki leaf | Temperature: 25–95 °C | Spherical and plate; 2–20 nm | [72] | |
| Ocimum sanctum leaf | Temperature: 100 °C; duration: 1 h | Rectangular and triangular; 23 nm | [73] | |
| Copper (Cu) | Mulberry fruit (Morus alba L.) | Temperature: ambient; duration: 5 h | Spherical and non–regular; 50–200 nm | [74] |
| Crotalaria candicans leaf | - | Spherical; 30 nm | [75] | |
| Ziziphus spinachristi fruit | Temperature: 80 °C | Spherical; 5–20 nm | [76] | |
| Clove (Syzygium aromaticum) buds | Temperature: 30 °C; duration: 15 min | Spherical; 15–20 nm | [77] | |
| Iron (Fe) | Tea leaves extract | Temperature: 80 °C; duration: 3 h | 30–100 nm | [78] |
| Moringa oleifera seeds | Temperature: ambient; duration: 30 min | Spherical; 2.6–6.2 nm | [79] | |
| Trigonella foenum–graecum seed | Temperature: 30 °C; duration: 5 min | 7–14 nm | [80] | |
| Selenium (Se) | Ocimum tenuiflorum | Temperature: ambient; duration: 75 h | Monodispersed and spherical; 15–20 nm | [81] |
| Murraya koenigii | - | Spherical; 50–150 nm | [82] | |
| Zinziber officinale fruit | Temperature: ambient; pH: 9.0; duration: 75 h | Spherical; 100–150 nm | [83] | |
| Nickel (Ni) | Calotropis gigantea leaves | Temperature: 80 °C; pH: 12.0; duration: 90 min | 60 nm | [84] |
| Desmodium gangeticum roots | Temperature: 80 °C; duration: 45 min | - | [85] |
| Microorganisms | Nanoparticles | Shape and Size | References | |
|---|---|---|---|---|
| Bacteria | ||||
| Bacillus subtilis | Ag | Spherical; 3–20 nm | [93] | |
| Pseudomonas stutzeri | Ag | Triangular; 200 nm | [94] | |
| Bacillus licheniformis | Ag | 40 nm | [95] | |
| Ochrobactrum anhtropi | Ag | Spherical; 38–85 nm | [96] | |
| Pantoea ananatis | Ag | Spherical; 8.06–91.31 nm | [97] | |
| Actinobacter | Ag | Spherical; 13.2 nm | [98] | |
| Pseudomonas aeruginosa | Au | 15–30 nm | [99] | |
| Rhodopseudomonas capsulata | Au | Spherical; 10–20 nm | [100] | |
| Escherichia coli DH5α | Au | Spherical, triangles, and quasi–hexagons; 25 nm | [101] | |
| Bacillus subtilis | Au | Spherical, 20–25 nm | [102] | |
| Mycobacterium sp. | Au | Spherical; 5–55 nm | [103] | |
| Shewanella loihica | Pt | 1–10 nm | [104] | |
| Shewanella oneidensis MR–1 | Pt | 2.83–61.03 nm | [105] | |
| Jeotgalicoccus coquinae ZC15 | Pt | Spherical; 5.74 nm | [106] | |
| Shewanella loihica | Pd | 1–12 nm | [104] | |
| Shewanella oneidensis MR–1 | Pd | 10–100 nm | [107] | |
| Lysinibacillus sp. ZYM-1 | Se | Cubic; 100–200 nm | [108] | |
| Bacillus subtilis | Se | Spherical; 50–400 nm | [109] | |
| Lactobacillus acidophilus | Se | Spherical; 2–15 nm | [110] | |
| Fungi | ||||
| Rhizopus stolonifer | Ag | Spherical; 2.86 nm | [111] | |
| Candida glabrata | Ag | Spherical; 2–15 nm | [112] | |
| Trametes trogii | Ag | Spherical and rod; 5–65 nm | [113] | |
| Trichoderma longibrachiatum | Ag | Spherical; 10 nm | [114] | |
| Fusarium oxysporum | Ag | Spherical; 21.3–37 nm | [115] | |
| Aspergillus terreus | Ag | Spherical; 7–23 nm | [116] | |
| Ganoderma sessiliforme | Ag | Spherical; 45 nm | [117] | |
| Candida albicans ATCC 10231 | Ag | Spherical; 10–20 nm | [118] | |
| Cladosporium cladosporioides | Au | 60 nm | [119] | |
| Trichoderma harzianum | Au | Spherical; 26–34 nm | [120] | |
| Pleurotus ostreatus | Au | Spherical; 10–30 nm | [121] | |
| Aspergillus sp. | Au | Spherical; 4–29 nm | [122] | |
| Rhizopus oryzae | Au | Spherical and flower like structure; 16–43 nm | [123] | |
| Penicillium chrysogenum | Pt | Spherical; 5–40 nm | [124] | |
| Fusarium oxysporum f. sp. lycopersici | Pt | Triangle, hexagons, square, and rectangles; 10–50 nm | [125] | |
| Fusarium oxysporum | Si | Quasi–spherical; 5–15 nm | [126] | |
| Fusarium oxysporum | Ti | Spherical; 6–13 nm | [126] | |
| Yeast | ||||
| Rhodotorula sp. ATL72 | Ag | Spherical and oval; 8–21 nm | [127] | |
| Saccharomyces cerevisiae | Ag | Spherical; 2–20 nm | [128] | |
| Cryptococcus laurentii | Ag | 35–400 nm | [129] | |
| Rhodotorula glutinis | Ag | 15–220 nm | [129] | |
| Rhodotorula glutinis | Ag | Spherical; 15.5 nm | [130] | |
| Saccharomyces cerevisiae | Au | Triangle, truncated triangle, and hexagon | [131] | |
| Magnusiomyces ingens LHF1 | Au | Spherical and pseudo–spherical; 20–28 nm | [132] | |
| Saccharomyces cerevisiae | Pd | Hexagonal; 32 nm | [133] | |
| Magnusiomyces ingens LHF1 | Se | Spherical and quasi–spherical; 70–90 nm | [134] | |
| Application | NPs/Nanotechnology | Function | Reference |
|---|---|---|---|
| Food Production | TiO2 | Antimicrobial, coating in packaging material, detection of volatile compounds | [198] |
| Nanoemulsion | Quality enhancement of beverages, sweeteners, and processed food | [195,196,197] | |
| Nanoencapsulation | Enhancement of taste, color, and odor of food materials | [194] | |
| Food preservation and packaging | AgNPs, Ag–ZnO NPs | Packaging of meat, fruit, and dairy products by AgNPs—doped nondegradable and edible polymers and oils; antimicrobial property | [199] |
| Low-density polyethylene film + Ag, ZnO NPs, TiO2, kaolin | Orange juice, blueberry, strawberry | [200,201,202] | |
| Ethylene vinyl alcohol + AgNPs | Chicken, pork, cheese, lettuce, apples, peels, eggshells | [203] | |
| Polyvinylchloride + AgNPs | Minced beef | [204] | |
| Polyethylene + Ag, TiO2 NPs | Fresh apples, white sliced bread, fresh carrots, soft cheese, atmosphere packaging milk powder, fresh orange juice | [205,206] | |
| Nanoclay-polymer nanocomposites | Meats, cheese, confectionery, cereals, boil-in-the-bag foods, extrusion-coating applications for fruit juices and dairy products, bottles for beer and carbonated drinks | [207] | |
| Ag-ZnO NPs | Nanostorage containers, bakeware, containers, cutting boards | [199] | |
| ZnNPs | Preservation and transport | [208] | |
| Food supplement and value addition | Colloidal metal nanoparticles | Enhanced uptake | - |
| Nanopowders | Increase absorption of nutrients | - | |
| Cellulose nanocrystal composites | Drug carrier | - | |
| Nanocochleates | Drug delivery, enhancement of taste and color of food materials | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. https://doi.org/10.3390/catal11080902
Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, Gupta PK, Kim BS. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts. 2021; 11(8):902. https://doi.org/10.3390/catal11080902
Chicago/Turabian StyleDikshit, Pritam Kumar, Jatin Kumar, Amit K. Das, Soumi Sadhu, Sunita Sharma, Swati Singh, Piyush Kumar Gupta, and Beom Soo Kim. 2021. "Green Synthesis of Metallic Nanoparticles: Applications and Limitations" Catalysts 11, no. 8: 902. https://doi.org/10.3390/catal11080902
APA StyleDikshit, P. K., Kumar, J., Das, A. K., Sadhu, S., Sharma, S., Singh, S., Gupta, P. K., & Kim, B. S. (2021). Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts, 11(8), 902. https://doi.org/10.3390/catal11080902