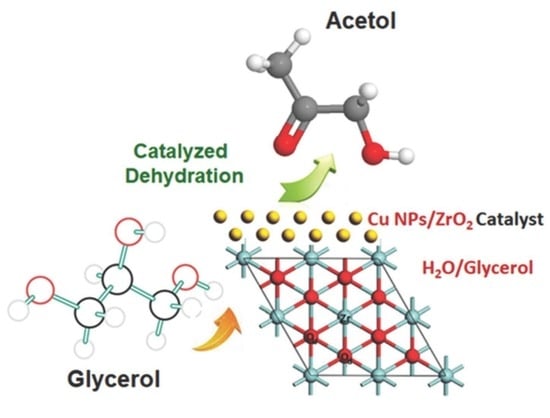
Glycerol Valorization over ZrO2-Supported Copper Nanoparticles Catalysts Prepared by Chemical Reduction Method
Abstract
:1. Introduction
2. Results
2.1. Copper NPs Characterization
2.2. UV–Visible Spectroscopy of Cu-NPs
2.3. Copper Catalyst Characterization
2.4. Catalyst Activity
2.5. Selectivity and Yield to Acetol
2.6. General Chemical Route
3. Discussion
4. Materials and Methods
4.1. Copper Nanoparticles Synthesis
4.2. Zirconia-Supported Copper Nanoparticles Catalysts
4.3. Copper Materials Characterization
4.3.1. Cu Nanoparticles (NPs)
4.3.2. Zirconia-Supported Copper Nanoparticles Catalysts
4.3.3. Glycerol Dehydration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jefferson, M. Sustainable energy development: Performance and prospects. Renew. Energy 2006, 31, 571–582. [Google Scholar] [CrossRef]
- Cadenas, A.; Cabezudo, S. Biofuels as Sustainable Technologies: Perspectives for Less Developed Countries. Technol. Forecast. Soc. Chang. 1998, 58, 83–103. [Google Scholar] [CrossRef]
- Türe, S.; Uzun, D.; Türe, I.E. The potential use of sweet sorghum as a non-polluting source of energy. Energy 1997, 22, 17–19. [Google Scholar] [CrossRef]
- Sheehan, J.; Camobreco, V.; Duffield, J.; Graboski, M.; Shapouri, H. An Overview of Biodiesel and Petroleum Diesel Life Cycles; U.S. Department of Energy (DOE) and and the U.S. Department of Agriculture (USDA): Golden, CO, USA, 1998.
- Demirbas, A. Progress and recent trends in biofuels. Prog. Energy Combust. Sci. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Rempel, G.L.; Ng, F.T.T. The Promoting Effect of Ni on Glycerol Hydrogenolysis to 1,2-Propanediol with In Situ Hydrogen from Methanol Steam Reforming Using a Cu/ZnO/Al2O3 Catalyst. Catalysts 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Mitta, H.; Seelam, P.K.; Ojala, S.; Keiski, R.L.; Balla, P. Tuning Y-zeolite based catalyst with copper for enhanced activity and selectivity in vapor phase hydrogenolysis of glycerol to 1,2-propanediol. Appl. Catal. A Gen. 2018, 550, 308–319. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Umeta, M.; Faulks, R.M. The effect of fermentation on the carbohydrates in tef (Eragrostis tef). Food Chem. 1988, 27, 181–189. [Google Scholar] [CrossRef]
- Yin, X.; Leung, D.Y.C.; Chang, J.; Wang, J.; Fu, Y.; Wu, C. Characteristics of the Synthesis of Methanol Using Biomass-Derived Syngas. Energy Fuels 2005, 19, 305–310. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Hisham, M.W.M.; Yarmo, M.A.; Yun Hin, T. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- Barakos, N.; Pasias, S.; Papayannakos, N. Transesterification of triglycerides in high and low quality oil feeds over an HT2 hydrotalcite catalyst. Bioresour. Technol. 2008, 99, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Biodiesel from vegetable oils via transesterification in supercritical methanol. Energy Convers. Manag. 2002, 43, 2349–2356. [Google Scholar] [CrossRef]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Brønsted Acid Sites in the Dehydration of Glycerol over Niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Azri, N.; Ramli, I.; Nda-Umar, U.I.; Shamsuddin, M.R.; Saiman, M.I.; Taufiq-Yap, Y.H. Copper-dolomite as effective catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Taiwan Inst. Chem. Eng. 2020, 112, 34–51. [Google Scholar] [CrossRef]
- Mitta, H.; Devunuri, N.; Sunkari, J.; Mutyala, S.; Balla, P.; Perupogu, V. A highly active dispersed copper oxide phase on calcined Mg9Al2.7-Ga2.3O2 catalysts in glycerol hydrogenolysis. Catal. Today 2021, 375, 204–215. [Google Scholar] [CrossRef]
- Mishra, N.K.; Kumar, P.; Srivastava, V.C.; Štangar, U.L. Synthesis of Cu-based catalysts for hydrogenolysis of glycerol to 1,2-propanediol with in-situ generated hydrogen. J. Environ. Chem. Eng. 2021, 9, 105263. [Google Scholar] [CrossRef]
- Alhanash, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt. Appl. Catal. A Gen. 2010, 378, 11–18. [Google Scholar] [CrossRef]
- Basu, S.; Shree, V.; Sen, A.K. Role of cerium as a promoter and process optimization studies for dehydration of glycerol to acetol over copper chromite catalyst. J. Rare Earths 2021. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Xiao, G. Sustainable value-added C3 chemicals from glycerol transformations: A mini review for heterogeneous catalytic processes. Chin. J. Chem. Eng. 2019, 27, 1536–1542. [Google Scholar] [CrossRef]
- Suganuma, S.; Hisazumi, T.; Taruya, K.; Tsuji, E.; Katada, N. Influence of Acidic Property on Catalytic Activity and Selectivity in Dehydration of Glycerol. ChemistrySelect 2017, 2, 5524–5531. [Google Scholar] [CrossRef]
- Yun, D.; Yun, Y.S.; Kim, T.Y.; Park, H.; Lee, J.M.; Han, J.W.; Yi, J. Mechanistic study of glycerol dehydration on Brønsted acidic amorphous aluminosilicate. J. Catal. 2016, 341, 33–43. [Google Scholar] [CrossRef]
- Suprun, W.; Lutecki, M.; Haber, T.; Papp, H. Acidic catalysts for the dehydration of glycerol: Activity and deactivation. J. Mol. Catal. A Chem. 2009, 309, 71–78. [Google Scholar] [CrossRef]
- Gabrysch, T.; Peng, B.; Bunea, S.; Dyker, G.; Muhler, M. The Role of Metallic Copper in the Selective Hydrodeoxygenation of Glycerol to 1,2-Propanediol over Cu/ZrO2. ChemCatChem 2018, 10, 1344–1350. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Requies, J.; Güemez, M.B.; Fierro, J.L.G. Hydrogenolysis of glycerol to propanediols over a Pt/ASA catalyst: The role of acid and metal sites on product selectivity and the reaction mechanism. Appl. Catal. B Environ. 2010, 97, 248–256. [Google Scholar] [CrossRef]
- Maris, E.P.; Davis, R.J. Hydrogenolysis of glycerol over carbon-supported Ru and Pt catalysts. J. Catal. 2007, 249, 328–337. [Google Scholar] [CrossRef]
- Mazarío, J.; Concepción, P.; Ventura, M.; Domine, M.E. Continuous catalytic process for the selective dehydration of glycerol over Cu-based mixed oxide. J. Catal. 2020, 385, 160–175. [Google Scholar] [CrossRef]
- Célerier, S.; Morisset, S.; Batonneau-Gener, I.; Belin, T.; Younes, K.; Batiot-Dupeyrat, C. Glycerol dehydration to hydroxyacetone in gas phase over copper supported on magnesium oxide (hydroxide) fluoride catalysts. Appl. Catal. A Gen. 2018, 557, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Azri, N.; Irmawati, R.; Nda-Umar, U.I.; Saiman, M.I.; Taufiq-Yap, Y.H. Effect of different supports for copper as catalysts on glycerol hydrogenolysis to 1,2-propanediol. J. King Saud Univ.-Sci. 2021, 33, 101417. [Google Scholar] [CrossRef]
- Freitas, I.C.; Manfro, R.L.; Souza, M.M.V.M. Hydrogenolysis of glycerol to propylene glycol in continuous system without hydrogen addition over Cu-Ni catalysts. Appl. Catal. B Environ. 2018, 220, 31–41. [Google Scholar] [CrossRef]
- Chimentão, R.J.; Hirunsit, P.; Torres, C.S.; Ordoño, M.B.; Urakawa, A.; Fierro, J.L.G.; Ruiz, D. Selective dehydration of glycerol on copper based catalysts. Catal. Today 2021, 367, 58–70. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.S.; Cheong, K.Y. Advances of Ag, Cu, and Ag–Cu alloy nanoparticles synthesized via chemical reduction route. J. Nanoparticle Res. 2013, 15, 1537. [Google Scholar] [CrossRef]
- Tamilvanan, A.; Balamurugan, K.; Ponappa, K.; Kumar, B.M. Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application. Int. J. Nanosci. 2014, 13, 1430001. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P.; Patel, B.H. Characterization and Stabilization of Nanosized Copper Particles. Int. J. Pure Appl. Sci. Technol. 2012, 9, 1–8. [Google Scholar]
- Rajesh, K.M.; Ajitha, B.; Ashok Kumar Reddy, Y.; Suneetha, Y.; Sreedhara Reddy, P. Synthesis of copper nanoparticles and role of pH on particle size control. Mater. Today Proc. 2016, 3, 1985–1991. [Google Scholar] [CrossRef]
- Chandra, S.; Kumar, A.; Tomar, P.K. Synthesis and characterization of copper nanoparticles by reducing agent. J. Saudi Chem. Soc. 2014, 18, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Dung Dang, T.M.; Tuyet Le, T.T.; Fribourg-Blanc, E.; Chien Dang, M. The influence of solvents and surfactants on the preparation of copper nanoparticles by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 25004. [Google Scholar] [CrossRef]
- Peng, C.; Shen, C.; Zheng, S.; Yang, W.; Hu, H.; Liu, J.; Shi, J. Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomater 2017, 7, 326. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.F.G. Nanoparticles in Catalysis. Top. Catal. 2003, 24, 147–159. [Google Scholar] [CrossRef]
- Brunelle, J.P. Preparation of Catalysts by metallic complex adsorption on mineral oxides. Pure Appl. Chem. 1978, 50, 1211–1229. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Salavati-Niasari, M.; Davar, F. Synthesis of copper and copper(I) oxide nanoparticles by thermal decomposition of a new precursor. Mater. Lett. 2009, 63, 441–443. [Google Scholar] [CrossRef]
- Merkus, H.G. Particle Size Measurements: Fundamentals, Practice, Quality, 1st ed.; Springer Science, Ed.; Particle Technology Series; Springer: Dordrecht, The Netherlands, 2009; ISBN 9781402090165. [Google Scholar]
- Chary, K.V.R.; Sagar, G.V.; Srikanth, C.S.; Rao, V.V. Characterization and Catalytic Functionalities of Copper Oxide Catalysts Supported on Zirconia. J. Phys. Chem. B 2007, 111, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Souma, Y.; Kawasaki, H. Synthesis of tert.-Alkanoic acid catalyzed by Cu(CO)n+ and Ag(CO)2+ under atmospheric pressure. Catal. Today 1997, 36, 91–97. [Google Scholar] [CrossRef]
- Tada, S.; Katagiri, A.; Kiyota, K.; Honma, T.; Kamei, H.; Nariyuki, A.; Uchida, S.; Satokawa, S. Cu Species Incorporated into Amorphous ZrO2 with High Activity and Selectivity in CO2-to-Methanol Hydrogenation. J. Phys. Chem. C 2018, 122, 5430–5442. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J. Cu/Al2O3 catalysts for soot oxidation: Copper loading effect. Appl. Catal. B Environ. 2008, 84, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Águila, G.; Gracia, F.; Araya, P. CuO and CeO2 catalysts supported on Al2O3, ZrO2, and SiO2 in the oxidation of CO at low temperature. Appl. Catal. A Gen. 2008, 343, 16–24. [Google Scholar] [CrossRef]
- Shimokawabe, M.; Asakawa, H.; Takezawa, N. Characterization of copper/zirconia catalysts prepared by an impregnation method. Appl. Catal. 1990, 59, 45–58. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Kang, H.; Chen, J.; Zhang, X.; Xia, C. Highly Dispersed Silica-Supported Copper Nanoparticles Prepared by Precipitation−Gel Method: A Simple but Efficient and Stable Catalyst for Glycerol Hydrogenolysis. Chem. Mater. 2008, 20, 5090–5099. [Google Scholar] [CrossRef]
- Volanti, D.P.; Sato, A.G.; Orlandi, M.O.; Bueno, J.M.C.; Longo, E.; Andrés, J. Insight into Copper-Based Catalysts: Microwave-Assisted Morphosynthesis, In Situ Reduction Studies, and Dehydrogenation of Ethanol. ChemCatChem 2011, 3, 839–843. [Google Scholar] [CrossRef]
- Yin, M.; Wu, C.-K.; Lou, Y.; Burda, C.; Koberstein, J.T.; Zhu, Y.; O’Brien, S. Copper Oxide Nanocrystals. J. Am. Chem. Soc. 2005, 127, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Sekar, K.; Chuaicham, C.; Balijapalli, U.; Li, W.; Wilson, K.F.; Lee, A.; Sasaki, K. Surfactant- and template-free hydrothermal assembly of Cu2O visible light photocatalysts for trimethoprim degradation. Appl. Catal. B Environ. 2021, 284, 119741. [Google Scholar] [CrossRef]
- Chang, X.; Liu, A.-F.; Cai, B.; Luo, J.-Y.; Pan, H.; Huang, Y.-B. Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran and 2-Methyltetrahydrofuran over Bimetallic Copper–Palladium Catalysts. ChemSusChem 2016, 9, 3330–3337. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, Y.; Zhou, H.; Wang, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Efficient Synthesis of Furfuryl Alcohol from H2-Hydrogenation/Transfer Hydrogenation of Furfural Using Sulfonate Group Modified Cu Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Beinik, I.; Hellström, M.; Jensen, T.N.; Broqvist, P.; Lauritsen, J.V. Enhanced wetting of Cu on ZnO by migration of subsurface oxygen vacancies. Nat. Commun. 2015, 6, 8845. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Wu, G.-S.; Wang, L.-C.; Liu, Y.-M.; Cao, Y.; Dai, W.-L.; He, H.-Y.; Fan, K.-N. Implication of the role of oxygen anions and oxygen vacancies for methanol decomposition over zirconia supported copper catalysts. Appl. Surf. Sci. 2006, 253, 974–982. [Google Scholar] [CrossRef]
- Tanabe, K.; Misono, M.; Hattori, H.; Ono, Y. New Solid Acids and Bases: Their Catalytic Properties; Elsevier Science: Amsterdam, The Netherlands, 1990; ISBN 9780080887555. [Google Scholar]
- Kim, Y.T.; Jung, K.-D.; Park, E.D. A comparative study for gas-phase dehydration of glycerol over H-zeolites. Appl. Catal. A Gen. 2011, 393, 275–287. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Atia, H. Recent developments in dehydration of glycerol toward acrolein over heteropolyacids. Eur. J. Lipid Sci. Technol. 2012, 114, 10–23. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.-P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Montassier, C.; Giraud, D.; Barbier, J. Polyol Conversion by Liquid Phase Heterogeneous Catalysis Over Metals. In Heterogeneous Catalysis and Fine Chemicals; Guisnet, M., Barrault, J., Bouchoule, C., Duprez, D., Montassier, C., Pérot, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 41, pp. 165–170. ISBN 0167-2991. [Google Scholar]
- Yfanti, V.-L.; Lemonidou, A.A. Mechanistic study of liquid phase glycerol hydrodeoxygenation with in-situ generated hydrogen. J. Catal. 2018, 368, 98–111. [Google Scholar] [CrossRef]
- Montassier, C.; Dumas, J.M.; Granger, P.; Barbier, J. Deactivation of supported copper based catalysts during polyol conversion in aqueous phase. Appl. Catal. A Gen. 1995, 121, 231–244. [Google Scholar] [CrossRef]
- Chimentão, R.J.; Miranda, B.C.; Szanyi, J.; Sepulveda, C.; Santos, J.B.O.; Correa, J.V.S.; Llorca, J.; Medina, F. Sources of deactivation during glycerol conversion on Ni/γ-Al2O3. Mol. Catal. 2017, 435, 49–57. [Google Scholar] [CrossRef]
- Xia, X.; Xie, C.; Cai, S.; Yang, Z.; Yang, X. Corrosion characteristics of copper microparticles and copper nanoparticles in distilled water. Corros. Sci. 2006, 48, 3924–3932. [Google Scholar] [CrossRef]
- Miranda, B.C.; Chimentão, R.J.; Szanyi, J.; Braga, A.H.; Santos, J.B.O.; Gispert-Guirado, F.; Llorca, J.; Medina, F. Influence of copper on nickel-based catalysts in the conversion of glycerol. Appl. Catal. B Environ. 2015, 166–167, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Wawrzetz, A.; Peng, B.; Hrabar, A.; Jentys, A.; Lemonidou, A.A.; Lercher, J.A. Towards understanding the bifunctional hydrodeoxygenation and aqueous phase reforming of glycerol. J. Catal. 2010, 269, 411–420. [Google Scholar] [CrossRef]
- Roy, D.; Subramaniam, B.; Chaudhari, R.V. Aqueous phase hydrogenolysis of glycerol to 1,2-propanediol without external hydrogen addition. Catal. Today 2010, 156, 31–37. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Guo, X.; Mao, J.; Zhang, S. Ag/Al2O3 for glycerol hydrogenolysis to 1,2-propanediol: Activity, selectivity and deactivation. Green Chem. 2012, 14, 156–163. [Google Scholar] [CrossRef]
- Furikado, I.; Miyazawa, T.; Koso, S.; Shimao, A.; Kunimori, K.; Tomishige, K. Catalytic performance of Rh/SiO2 in glycerol reaction under hydrogen. Green Chem. 2007, 9, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Durán-Martín, D.; Ojeda, M.; Granados, M.L.; Fierro, J.L.G.; Mariscal, R. Stability and regeneration of Cu–ZrO2 catalysts used in glycerol hydrogenolysis to 1,2-propanediol. Catal. Today 2013, 210, 98–105. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, L.; Xie, W.; Chen, P.; Hou, Z.; Zheng, X. Biodiesel derived glycerol hydrogenolysis to 1,2-propanediol on Cu/MgO catalysts. Bioresour. Technol. 2010, 101, 7088–7092. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Investigating the performance and deactivation behaviour of silica-supported copper catalysts in glycerol hydrogenolysis. Appl. Catal. A Gen. 2011, 396, 177–185. [Google Scholar] [CrossRef]
- Liu, Y.; Mai, C.T.Q.; Ng, F.T.T. Glycerol Hydrogenolysis with In Situ Hydrogen Produced via Methanol Steam Reforming: The Promoting Effect of Pd on a Cu/ZnO/Al2O3 Catalyst. Catalysts 2021, 11, 110. [Google Scholar] [CrossRef]
- Xia, S.; Zheng, L.; Wang, L.; Chen, P.; Hou, Z. Hydrogen-free synthesis of 1,2-propanediol from glycerol over Cu–Mg–Al catalysts. RSC Adv. 2013, 3, 16569–16576. [Google Scholar] [CrossRef]
- Seguel, J.; García, R.; Chimentão, R.J.; García-Fierro, J.L.; Ghampson, I.T.; Escalona, N.; Sepúlveda, C. Thermal Modification Effect on Supported Cu-Based Activated Carbon Catalyst in Hydrogenolysis of Glycerol. Materials 2020, 13, 603. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Li, S.; Yu, L.; Liu, Y.; Cao, Y. Efficient catalytic hydrogenolysis of glycerol using formic acid as hydrogen source. Chin. J. Catal. 2013, 34, 2066–2074. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, C.; Yin, H.; Gao, D.; Shen, L.; Wang, A. Hydrothermal conversion of glycerol to lactic acid catalyzed by Cu/hydroxyapatite, Cu/MgO, and Cu/ZrO2 and reaction kinetics. Chem. Eng. J. 2016, 288, 332–343. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Schüth, F.; Weitkamp, J. Handbook Of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2008; Volume 1, ISBN 978-3-527-31241-2. [Google Scholar]








| Sample | Specific Surface Area SBET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Cu NPs/ZrO2 pH = 4 | 129 | 0.131 | 4.1 |
| Cu NPs/ZrO2 pH = 7 | 134 | 0.137 | 4.1 |
| Cu NPs/ZrO2 pH = 9 | 136 | 0.143 | 4.2 |
| ZrO2 | 146 | 0.156 | 4.3 |
| Sample | Tmax (°C) 1 | Tmax (°C) 2 | NH3 (mmol/gcat) 3 | N2O (µmol/gcat) 4 | Copper (g Cu/gcat) 5 | D (%) | |
|---|---|---|---|---|---|---|---|
| Cu NPs/ZrO2 pH = 4 | 153.7 | – | 1.3 | 151 | 0.0191 | 54.8 | 1.8 |
| Cu NPs/ZrO2 pH = 7 | 163.5 | – | 1.1 | 149 | 0.0189 | 54.1 | 1.8 |
| Cu NPs/ZrO2 pH = 9 | 161.4 | 206.3 | 1.5 | 116 | 0.0148 | 42.3 | 2.3 |
| Material | Atomic Surface % | Cu 2p/Zr 3d Atomic Ratio a | |||
|---|---|---|---|---|---|
| C 1s | O 1s | Zr 3d | Cu 2p | ||
| Cu NPs/ZrO2 pH = 4 | 14.17 | 63.38 | 20.40 | 2.05 | 0.100 |
| Cu NPs/ZrO2 pH = 7 | 11.18 | 65.98 | 20.87 | 1.97 | 0.094 |
| Cu NPs/ZrO2 pH = 9 | 13.43 | 64.62 | 20.08 | 1.88 | 0.093 |
| Sample | Cu 2p3/2 B.E. (eV) | Cu LMM K.E. (eV) | Modified Auger Parameter a |
|---|---|---|---|
| Cu NPs/ZrO2 pH = 4 | 932.88 | 916.78 | 1849.66 |
| Cu NPs/ZrO2 pH = 7 | 933.38 | 915.75 | 1849.13 |
| Cu NPs/ZrO2 pH = 9 | 933.05 | 917.61 | 1850.66 |
| Sample | k (min−1) |
|---|---|
| Cu NPs/ZrO2 pH = 4 | 0.0013 |
| Cu NPs/ZrO2 pH = 7 | 0.0013 |
| Cu NPs/ZrO2 pH = 9 | 0.0040 |
| Catalysts | T (°C) | H2 Pressure (atm) | Glycerol/Catalyst (g/g) | Glycerol Conversion (%) | Time (h) | Selectivity to1,2–PDO (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Ag/Al2O3 | 220 | 15.0 | 7.6 | 46.0 | 10 | 96.0 | [75] |
| Rh/SiO2 | 120 | 80.0 | 27.8 | 19.6 | 10 | 34.6 | [76] |
| Pt/SiO2-Al2O3 | 220 | 45.0 | 6.0 | 6.0 | 24 | 31.9 | [27] |
| Cu/Dolomite | 200 | 40.0 | 20.0 | 78.5 | 10 | 79.0 | [17] |
| Cu/ZrO2 | 200 | 40.0 | 41.6 | 12.8 | 8 | 12.8 | [77] |
| Cu/MgO | 180 | 30.0 | 7.1 | 72.0 | 20 | 97.6 | [78] |
| Cu/SiO2 | 240 | 78.9 | 166.0 | 4.1 | 5 | 90.1 | [79] |
| Cu/ZrO2 | 190 | --- a | 160.0 | 40.0 | 3 | 10.0 | This work |
| Cu/ZnO/Al2O3 | 220 | --- a | 100.0 | 70.2 | 6 | 63.0 | [80] |
| Cu0.4/Mg5.6Al2O8.6 | 220 | --- a | 8.0 | 33.1 | 10 | 91.7 | [81] |
| Cu/C | 220 | 50 | 150.0 | 16.6 | 6 | 78.6 | [82] |
| Cu/ZrO2 | 200 | 80 | 10.0 | 85.0 | 5 | 94.0 | [83] |
| Cu/MgO | 230 | --- a | 20.0 | 90.0 | 2 | 1.0 | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcés, J.; Arrué, R.; Novoa, N.; Peixoto, A.F.; Chimentão, R.J. Glycerol Valorization over ZrO2-Supported Copper Nanoparticles Catalysts Prepared by Chemical Reduction Method. Catalysts 2021, 11, 1040. https://doi.org/10.3390/catal11091040
Garcés J, Arrué R, Novoa N, Peixoto AF, Chimentão RJ. Glycerol Valorization over ZrO2-Supported Copper Nanoparticles Catalysts Prepared by Chemical Reduction Method. Catalysts. 2021; 11(9):1040. https://doi.org/10.3390/catal11091040
Chicago/Turabian StyleGarcés, Juan, Ramón Arrué, Néstor Novoa, Andreia F. Peixoto, and Ricardo J. Chimentão. 2021. "Glycerol Valorization over ZrO2-Supported Copper Nanoparticles Catalysts Prepared by Chemical Reduction Method" Catalysts 11, no. 9: 1040. https://doi.org/10.3390/catal11091040
APA StyleGarcés, J., Arrué, R., Novoa, N., Peixoto, A. F., & Chimentão, R. J. (2021). Glycerol Valorization over ZrO2-Supported Copper Nanoparticles Catalysts Prepared by Chemical Reduction Method. Catalysts, 11(9), 1040. https://doi.org/10.3390/catal11091040