Removal of Tar Contents Derived from Lignocellulosic Biomass Gasification Facilities Using MgAl-LDH@clinoptilolite
Abstract
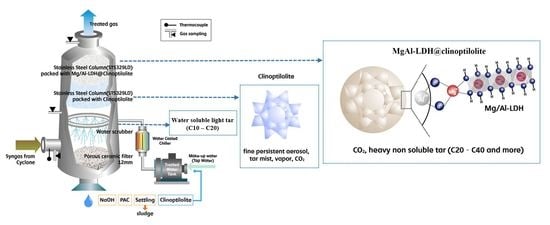
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Tar Production and Removal in Syngas
2.2. Reformation Characteristics of Inflammable Gas in Syngas
2.3. Processing and Recirculation of Tar Wastewater
3. Materials and Methods
3.1. Materials
3.2. Mg/Al-LDH@Clinoptilolite Preparation
3.3. Syngas Source and Tar Removal Process
3.4. Tar Sampling and Analysis Method
3.5. Analysis of Syngas
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The Government of the Republic of Korea. 2050 Carbon Neutral Strategy of The Republic of Korea: Towards a Sustainable and Green Society; The government of the Republic of Korea: Sejong-si, Korea, December 2020; pp. 15–48.
- The U.S. Energy Information Administration (EIA). Country Analysis Executive Summary: South Korea; EIA: Washington, DC, USA, October 2020; pp. 1–15. [Google Scholar]
- International Energy Agency. Global Energy Review 2021: Assessing the Effects of Economic Recoveries on Global Energy Demand and CO2 Emissions in 2021; International Energy Agency: Paris, France, 2021; pp. 1–32. [Google Scholar]
- Elliott, D.C. Relation of reaction time and temperature to chemical composition of pyrolysis oils. In Pyrolysis Oils from Biomass; Soltes, E.J., Milne, T.A., Eds.; ACS Publications: Washington, DC, USA, 1988; Volume 376, pp. 55–65. [Google Scholar]
- Milne, T.A.; Evans, R.J.; Abatzoglou, N. Biomass Gasifier ’’Tars’’: Their Nature, Formation, and Conversion; National Renewable Energy Laboratory: Golden, CO, USA, 1998. [Google Scholar]
- Phuphuakrat, T.; Namioka, T.; Yoshikawa, K. Tar removal from biomass pyrolysis gas in two-step function of decomposition and adsorption. Appl. Energy 2010, 87, 2203–2211. [Google Scholar] [CrossRef]
- Cateni, B.G. Effects of Feed Composition and Gasification Parameters on Product Gas from a Pilot Scale Fluidized Bed Gasifier. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2007. [Google Scholar]
- Coll, R.; Salvadó, J.; Farriol, X.; Montané, D. Steam reforming model compounds of biomass gasification tars: Conversion at different operating conditions and tendency towards coke formation. Fuel Process. Technol. 2001, 74, 19–31. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, X.; Lin, S.; Ji, X.; Bai, J.; Xu, M. Syngas production from air-steam gasification of biomass with natural catalysts. Sci. Total. Environ. 2018, 645, 518–523. [Google Scholar] [CrossRef]
- Rios, M.L.V.; González, A.M.; Lora, E.E.S.; del Olmo, O.A.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; van Paasen, S.V.B.; Boerrigter, H. The Novel ‘OLGA’ Technology for Complete Tar Removal from Biomass Producer Gas; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2002. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Han, J.; Kim, H. The reduction and control technology of tar during biomass gasification/pyrolysis: An overview. Renew. Sustain. Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Harba, R.; Rivera-Tinocoa, R.; Nemera, M.; Zeghondyb, B.; Boualloua, C. Process Simulation of Tar Removal from Gasification Producer Gas. Chem. Eng. 2020, 81, 931–936. [Google Scholar] [CrossRef]
- Nor Shafizah, I.; Salmiaton, A.; Arifin, N.A.; Hafriz, R.S.R.M.; Azlina, W.A.K.G.; Taufiq-Yap, Y.H.; Shamsuddin, A.H. H2-Rich and Tar-Free Downstream Gasification Reaction of EFB by Using the Malaysian Dolomite as a Secondary Catalyst. Catalysts 2021, 11, 447. [Google Scholar]
- Orío, A.; Corella, J.; Narváez, I. Performance of Different Dolomites on Hot Raw Gas Cleaning from Biomass Gasification with Air. Ind. Eng. Chem. Res. 1997, 36, 3800–3808. [Google Scholar] [CrossRef]
- Simell, P.A.; Hakala, A.N.A.K.; Haario, H.E.; Krause, A.O.I. Catalytic Decomposition of Gasification Gas Tar with Benzene as the Model Compound. Ind. Eng. Chem. Res. 1997, 36, 42–51. [Google Scholar] [CrossRef]
- Corella, J.; Aznar, M.-P.; Gil, J.; Caballero, M.A. Biomass Gasification in Fluidized Bed: Where to Locate the Dolomite to Improve Gasification? Energy Fuels 1999, 13, 1122–1127. [Google Scholar] [CrossRef]
- Delgado, J.; Aznar, M.P.; Corella, J. Calcined Dolomite, Magnesite, and Calcite for Cleaning Hot Gas from a Fluidized Bed Biomass Gasifier with Steam: Life and Usefulness. Ind. Eng. Chem. Res. 1996, 35, 3637–3643. [Google Scholar] [CrossRef]
- Narváez, I.; Orío, A.; Aznar, M.P.; Corella, J. Biomass Gasification with Air in an Atmospheric Bubbling Fluidized Bed. Effect of Six Operational Variables on the Quality of the Produced Raw Gas. Ind. Eng. Chem. Res. 1996, 35, 2110–2120. [Google Scholar] [CrossRef]
- Wang, T.; Chang, J.; Wu, C.; Fu, Y.; Chen, Y. The steam reforming of naphthalene over a nickel–dolomite cracking catalyst. Biomass Bioenergy 2005, 28, 508–514. [Google Scholar] [CrossRef]
- Devi, L.; Craje, M.; Thüne, P.; Ptasinski, K.J.; Janssen, F.J. Olivine as tar removal catalyst for biomass gasifiers: Catalyst characterization. Appl. Catal. A Gen. 2005, 294, 68–79. [Google Scholar] [CrossRef]
- Rapagnà, S.; Jand, N.; Kiennemann, A.; Foscolo, P. Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- Yung, M.M.; Jablonski, W.S.; Magrini-Bair, K.A. Review of Catalytic Conditioning of Biomass-Derived Syngas. Energy Fuels 2009, 23, 1874–1887. [Google Scholar] [CrossRef]
- Corella, J.; Toledo, A.J.M.; Padilla, R. Olivine or Dolomite as In-Bed Additive in Biomass Gasification with Air in a Fluidized Bed: Which Is Better? Energy Fuels 2004, 18, 713–720. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J. Pretreated olivine as tar removal catalyst for biomass gasifiers: Investigation using naphthalene as model biomass tar. Fuel Process. Technol. 2005, 86, 707–730. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Klerk, A. Zeolites as Catalysts for Fuels Refining after Indirect Liquefaction Processes. Molecules 2018, 23, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, B.L.F.; Gorin, A.; Chua, H.B.; Twaiq, F. Experimental investigation on tar produced from palm shells derived syngas using zeolite HZSM-5 catalyst. J. Energy Inst. 2016, 89, 713–724. [Google Scholar] [CrossRef]
- Dou, B.; Gao, J.; Sha, X.; Baek, S.W. Catalytic cracking of tar component from high-temperature fuel gas. Appl. Therm. Eng. 2003, 23, 2229–2239. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, S.; Han, T.U.; Park, Y.K.; Watanabe, C. Pyrolysis reaction characteristics of Korean pine (Pinus Koraiensis) nut shell. J. Anal. Appl. Pyrolysis 2014, 110, 435–441. [Google Scholar] [CrossRef]
- Kim, I.T. Treatment of Wood Tar Containing Wastewater Generated from Wood Composite Gasification Plant Using Mg/Al-LDH (Layered Double Hydroxides) Impregnated Natural Zeolite (Clinoptilolite). J. Water Treat. 2017, 25, 57–66. [Google Scholar]
- Xiao, R.; Zhang, M.; Jin, B.; Huang, Y.; Zhou, H. High-temperature air/steam-blown gasification of coal in a pressurized spout-fluid bed. Energy Fuels 2006, 20, 715–720. [Google Scholar] [CrossRef]
- Saw, W.L.; Pang, S. Co-gasification of blended lignite and wood pellets in a 100 kW dual fluidised bed steam gasifier: The influence of lignite ratio on producer gas composition and tar content. Fuel 2013, 112, 117–124. [Google Scholar] [CrossRef]
- Davarpanah, E.; Armandi, M.; Hernández, S.; Fino, D.; Arletti, R.; Bensaid, S.; Piumetti, M. CO2 capture on natural zeolite clinoptilolite: Effect of temperature and role of the adsorption sites. J. Environ. Manag. 2020, 275, 111229. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 adsorption capacities in zeolites and layered double hydroxide materials. Front. Chem. 2019, 7, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murge, P.; Dinda, S.; Roy, S. Zeolite-based sorbent for CO2 capture: Preparation and performance evaluation. Langmuir 2019, 35, 14751–14760. [Google Scholar] [CrossRef]
- Filippis, P.; Scarsella, M.; Caprariis, B.; RUccellari, R. Biomass gasification plant and syngas clean-up system. Energy Procedia 2015, 75, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Cali, G.; Deiana, P.; Maggio, E.; Marotto, D.; Mascia, M.; Vacca, A. Management and treatment of the clean-up water from the scrubber of a coal and biomass gasification plant: An industrial case study. Chem. Eng. Trans. 2019, 74, 337–342. [Google Scholar]
- Sajjadi, S.A.; Meknati, A.; Lima, E.C.; Dotto, G.L.; Mendoza-Castillo, D.I.; Anastopoulos, I.; Alakhras, F.; Unuabonah, E.I.; Singh, P.; Hosseini-Bandegharaei, A. A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb (II) sorption. J. Environ. Manag. 2019, 236, 34–44. [Google Scholar] [CrossRef]
- Heilmann, H.M.; Wiesmann, U.; Stenstrom, M.K. Kinetics of the alkaline hydrolysis of high explosives RDX and HMX in aqueous solution and adsorbed to activated carbon. Environ. Sci. Technol. 1996, 30, 1485–1492. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, W.; Zhu, H.; Yuan, P.; Yang, C.; Li, C.; Bao, X. Insights into the reaction pathway of n-butane conversion over HZSM-5 zeolite at low temperature. Appl. Catal. A-Gen. 2019, 584, 117135. [Google Scholar] [CrossRef]
- Mfoumou, C.M.; Mignard, S.; Belin, T. The preferential adsorption sites of H2O on adsorption sites of CO2 at low temperature onto NaX and BaX zeolites. Adsorp. Sci. Technol. 2018, 36, 1246–1259. [Google Scholar] [CrossRef] [Green Version]
- Favvas, E.P.; Tsanaktsidis, C.G.; Sapalidis, A.A.; Tzilantonis, G.T.; Papageorgiou, S.K.; Mitropoulos, A.C. Clinoptilolite, a natural zeolite material: Structural characterization and performance evaluation on its dehydration properties of hydrocarbon-based fuels. Microporous Mesoporous Mater. 2016, 225, 385–391. [Google Scholar] [CrossRef]
- Kim, I.T. Ammonium and nitrate removal characteristics of natural zeolite (Clinoptilolite) in the artificial seawater. J. Water Treat. 2014, 22, 23–30. [Google Scholar]
- Wang, X.; Zhu, X.; Meng, X. Preparation of a Mg/Al/Fe layered supramolecular compound and application for removal of Cr (VI) from laboratory wastewater. RSC Adv. 2017, 7, 34984–34993. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Song, Z.; Dou, Y.; Xue, Y.; Ji, Y.; Tang, Y.; Hu, M. Removal difference of Cr (VI) by modified zeolites coated with MgAl and ZnAl-layered double hydroxides: Efficiency, factors and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126583. [Google Scholar] [CrossRef]
- van de Kamp, W.L.; de Wild, P.J.; Knoef, H.A.M.; Neeft, J.P.A.; Kiel, J.H.A. Sampling and Analysis of Tar and Particles in Biomass Producer Gases; Report ECN-C—06-046; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2005. [Google Scholar]
- Dufour, A.; Girods, P.; Masson, E.; Normand, S.; Rogaume, Y.; Zoulalian, A. Comparison of two methods of measuring wood pyrolysis tar. J. Chromatogr. A 2007, 1164, 240–247. [Google Scholar] [CrossRef] [PubMed]









| Compounds | CAS No | Detected Mass-to-Charge Ratio (m/z) | % |
|---|---|---|---|
| Carbohydrates | 7.3 | ||
| Corylone | 80-71-7 | 112, 69, 55 | 5.4 |
| levoglucosan | 498-07-7 | 60, 57, 73 | 1.9 |
| Furans | 1.2 | ||
| (5H)-furan-2-one | 497-23-4 | 55, 84, 27 | 1.20 |
| Phenols | 35.6 | ||
| Phenol | 108-95-2 | 94, 66, 65 | 9.1 |
| o-cresol | 95-48-7 | 108, 107, 79 | 4.8 |
| m- or p-cresol | 108-39-4 | 108, 107, 79 | 8.6 |
| 2,6-dimethylphenol | 576-26-1 | 122, 107, 77 | 3.4 |
| 3-ethylphenol | 620-17-7 | 107, 122, 77 | 5.2 |
| catechol | 120-80-9 | 110, 64, 63 | 2.2 |
| 4-methylcatechol | 452-86-8 | 124, 123, 78 | 2.3 |
| Guaiacols | 41.5 | ||
| guaiacol | 90-05-1 | 109, 124, 81 | 11.2 |
| 2-methoxy-4-methylphenol | 93-51-6 | 138, 123, 95 | 1.9 |
| 4-ethylguaiacol | 2785-89-9 | 137, 152, 15 | 9.8 |
| 4-vinylguaiacol | 7786-61-0 | 135, 150, 107 | 2.7 |
| eugenol | 97-53-0 | 164, 103, 77 | 3.8 |
| vanillin | 121-33-5 | 152, 151, 81 | 4.4 |
| trans-isoeugenol | 97-54-1 | 164, 149, 103 | 1.5 |
| cis-isoeugenol | 164, 77, 149 | ||
| acetoguaiacone | 498-02-2 | 151, 166, 123 | 6.2 |
| No. | Chemical Species | Syngas from Cyclone | After Scrubber | After Mg/Al-LDH@Clinoptilolite | |
|---|---|---|---|---|---|
| mg/Nm3 | mg/Nm3 | mg/Nm3 | |||
| 1 | Carbohydrates | Corylone | 101.20 | 64.7 | 23.6 |
| 2 | levoglucosan | 12.34 | 7.9 | 2.1 | |
| 3 | Furans | (5H)-furan-2-one | 23.50 | 15.2 | 3.4 |
| 4 | Phenols | Phenol | 359.90 | 122.4 | 34.6 |
| 5 | o-cresol | 67.40 | 32.8 | 18.2 | |
| 6 | m- or p-cresol | 181.20 | 96.8 | 45.2 | |
| 7 | 2,6-dimethylphenol | 11.3 | 4.8 | 1.3 | |
| 8 | 3-ethylphenol | 76.90 | 45.2 | 13.6 | |
| 9 | catechol | 18.21 | 11.2 | 2.5 | |
| 10 | 4-methylcatechol | 21.38 | 8.9 | 1.8 | |
| 11 | Guaiacols | guaiacol | 31.30 | 18.1 | 9.4 |
| 12 | 2-methoxy-4-methylphenol | 58.90 | 43.2 | 12.6 | |
| 13 | 4-ethylguaiacol | 26.30 | 18.4 | 9.3 | |
| 14 | 4-vinylguaiacol | 152.10 | 78.9 | 34.2 | |
| 15 | eugenol | 38.20 | 26.28 | 6.7 | |
| 16 | vanillin | 61.40 | 15.4 | 8.4 | |
| 17 | trans-isoeugenol | 21.30 | 18.7 | 11.7 | |
| 18 | cis-isoeugenol | ||||
| 19 | acetoguaiacone | 87.60 | 48.9 | 23.8 | |
| Total | - | - | 1263.7 | 629.8 | 251.3 |
| Analysis Items | Raw Wastewater | NaOH + Ca(OH)2 Injection | PAC Injection | Zeolite (Clinoptilolite) |
|---|---|---|---|---|
| pH | 4.9 | 8.7 | 5.9 | 5.9 |
| Turbidity (NTU) | 41.7 | 129.0 | 12.7 | 8.8 |
| SS (mg·L−1) | 75.0 | 155.0 | 15.0 | 11.2 |
| CODcr (mg·L−1) | 2249.6 | 2136.8 | 1140.3 | 326.8 |
| NH4+-N(mg·L−1) | 31.2 | 22.4 | 15.8 | 2.6 |
| Chemical Component | Percent (%) | Chemical Component | Percent (%) |
|---|---|---|---|
| SiO2 Al2O8 Fe2O8 MgO CaO | 66.50 14.70 1.68 1.25 1.82 | Na2O K2O P2O5 H2O | 1.90 3.25 0.04 8.04 |
| Value | Unit | |
|---|---|---|
| C | 46.2 | w/w (%) |
| H | 6.1 | |
| O | 31.2 | |
| S | 0.01< | |
| N | 0.07 | |
| Cl | 0.03 | |
| Water | 17.2 | |
| Volatile Matter | 67.8 | |
| Fixed Carbon | 13.7 | |
| Non-volatile Matter | 1.3 | |
| Low Heating Value | 13.9 | MJ∙kg−1 |
| First Method (GC/MS) | Second Method (TD–GC/MS) | |
|---|---|---|
| Instrument | Perkin Elmer Clarus 600 GC/MS (Waltham, MA, USA)/(Detector: Clarus 600T) | GC/MS(TD–GC/MS), using a TurboMatrix thermal desorber (Perkin-Elmer) and a Clarus 600 GC/MS (Perkin-Elmer). (Detector: Clarus 600T) |
| Column | HP-5MS-UI, (5%-Phenyl)-methylpolysiloxane (Agilent J&W, Folsom, CA, USA) | |
| Carrier gas | Helium (Alphagaz 2, Air Liquide, Nancy, France), 1.2 mL/min | |
| GC oven | 40 °C, 5 min, 10 °C/min, 320 °C | |
| Inlets | Split less, Heater 250 °C | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.-T.; Ahn, K.-H.; Jung, J.; Jeong, Y.; Shin, D.-C.; Lee, Y.-E. Removal of Tar Contents Derived from Lignocellulosic Biomass Gasification Facilities Using MgAl-LDH@clinoptilolite. Catalysts 2021, 11, 1111. https://doi.org/10.3390/catal11091111
Kim I-T, Ahn K-H, Jung J, Jeong Y, Shin D-C, Lee Y-E. Removal of Tar Contents Derived from Lignocellulosic Biomass Gasification Facilities Using MgAl-LDH@clinoptilolite. Catalysts. 2021; 11(9):1111. https://doi.org/10.3390/catal11091111
Chicago/Turabian StyleKim, I-Tae, Kwang-Ho Ahn, Jinhong Jung, Yoonah Jeong, Dong-Chul Shin, and Ye-Eun Lee. 2021. "Removal of Tar Contents Derived from Lignocellulosic Biomass Gasification Facilities Using MgAl-LDH@clinoptilolite" Catalysts 11, no. 9: 1111. https://doi.org/10.3390/catal11091111
APA StyleKim, I. -T., Ahn, K. -H., Jung, J., Jeong, Y., Shin, D. -C., & Lee, Y. -E. (2021). Removal of Tar Contents Derived from Lignocellulosic Biomass Gasification Facilities Using MgAl-LDH@clinoptilolite. Catalysts, 11(9), 1111. https://doi.org/10.3390/catal11091111