Recent Advances in the Application of Enzyme Processing Assisted by Ultrasound in Agri-Foods: A Review
Abstract
:1. Introduction
2. Ultrasound Technology
2.1. Ultrasound Theoretical Basis
2.2. US Experimental Considerations
2.3. Ultrasound Devices
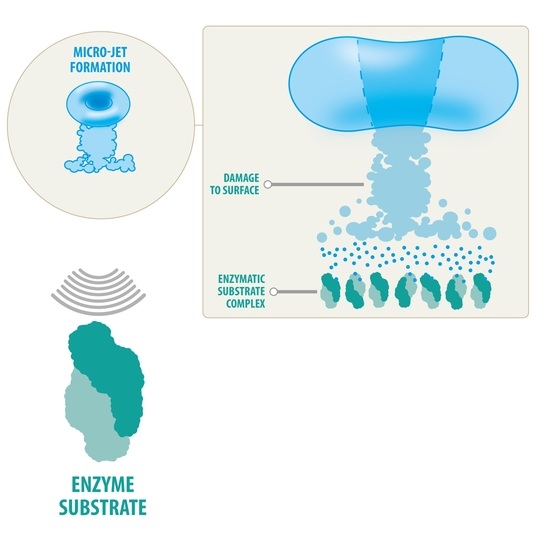
3. Effects of Ultrasound on Enzyme Structure
4. Enzyme Bioprocessing Assisted by Ultrasound in Agri-Foods
4.1. Ultrasound-Assisted Enzymatic Extractions
4.2. Enzymatic Hydrolysis Assisted by Ultrasound
4.3. Ultrasound in Biocatalysis
5. Future Trends and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Toldrá-Reig, F.; Toldrá, F. Use of enzymes to preserve food. Encycl. Food Secur. Sustain. 2018, 2, 511–517. [Google Scholar] [CrossRef]
- Kotzia, G.A.; Platis, D.; Axarli, I.A.; Chronopoulou, E.G.; Karamitros, C.; Labrou, N.E. Biocatalysis, Enzyme Engineering and Biotechnology. In Food Biochemistry and Food Processing: Second Edition; Simpson, B.K., Nollet, L.M.L., Toldra, F., Benjakul, S., Paliyath, G., Hui., Y.H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 125–166. ISBN 9780813808741. [Google Scholar]
- Fernandes, P. Enzymatic Processing in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Bilal, M.; Iqbal, H.M.N. State-of-the-art strategies and applied perspectives of enzyme biocatalysis in food sector - current status and future trends. Crit. Rev. Food Sci. Nutr. 2020, 60, 2052–2066. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Enzymes in Food Processing: A Condensed Overview on Strategies for Better Biocatalysts. Enzyme Res. 2010, 2010, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermelho, A.B.; Cardoso, V.; Nascimento, R.P.; Pinheiro, A.S.; Rodrigues, I.A. Application of Microbial Enzymes in the Food Industry. In Advances in Food Biotechnology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 103–132. [Google Scholar]
- Vera, C.; Guerrero, C.; Aburto, C.; Cordova, A.; Illanes, A. Conventional and non-conventional applications of β-galactosidases. Biochim. Biophys. Acta - Proteins Proteomics 2020, 1868, 140271. [Google Scholar] [CrossRef]
- Kuddus, M. Introduction to food enzymes. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Kuddus, M., Ed.; Academic Press: London, UK, 2018; pp. 1–18. ISBN 9780128132807. [Google Scholar]
- Illanes, A. Enzyme Biocatalysis: Principles and Applications; Illanes, A., Ed.; Springer: Valparaíso, Chile, 2008; ISBN 9781402083600. [Google Scholar]
- Homaei, A. Enzyme Immobilization and its Application in the Food Industry. In Advances in Food Biotechnology; John Wiley & Sons, Ltd.: New Jersey, USA, 2015; pp. 145–164. [Google Scholar]
- Agyei, D.; Shanbhag, B.K.; He, L. Enzyme engineering (immobilization) for food applications. Improv. Tailoring Enzym. Food Qual. Funct. 2015, 1984, 213–235. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, S.; Mokhtar, M.N.; Naim, M.N.; Baharuddin, A.S.; Sulaiman, A. A review: Potential usage of cellulose nanofibers (CNF) for enzyme immobilization via covalent interactions. Appl. Biochem. Biotechnol. 2015, 175, 1817–1842. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Schoevaart, R.; Van Langen, L.M. Cross-linked enzyme aggregates (CLEAs): A novel and versatile method for enzyme immobilization (a review). Biocatal. Biotransformation 2005, 23, 141–147. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, R.; Piacentini, E.; Gebreyohannes, A.Y.; Giorno, L. Membrane bioreactors in food, pharmaceutical and biofuel applications: State of the art, progresses and perspectives. Curr. Org. Chem. 2017, 21, 1671–1701. [Google Scholar] [CrossRef]
- Bashir, N.; Sood, M.; Bandral, J.D. Enzyme immobilization and its applications in food processing: A review. Int. J. Chem. Stud. 2020, 8, 254–261. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, M.; Yang, C. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.; Polachini, T.C.; Augusto, P.E.D.; Telis-Romero, J. Ultrasound-assisted hydration of wheat grains at different temperatures and power applied: Effect on acoustic field, water absorption and germination. Chem. Eng. Process. Process. Intensif. 2020, 155. [Google Scholar] [CrossRef]
- Yasui, K. Acoustic Cavitation and Bubble Dynamics; Pollet, B.G., Ashokkumar, M., Eds.; Srpinger: Cham, Switzerland, 2018; ISBN 978-3-319-68236-5. [Google Scholar]
- Córdova, A.; Astudillo-Castro, C.; Ruby-Figueroa, R.; Valencia, P.; Soto, C. Recent advances and perspectives of ultrasound assisted membrane food processing. Food Res. Int. 2020, 133, 109163. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Le, V.V.M. Effects of ultrasound on cellulolytic activity of cellulase complex. Int. Food Res. J. 2013, 20, 557–563. [Google Scholar]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Wang, D.; Yan, L.; Ma, X.; Wang, W.; Zou, M.; Zhong, J.; Ding, T.; Ye, X.; Liu, D. Ultrasound promotes enzymatic reactions by acting on different targets: Enzymes, substrates and enzymatic reaction systems. Int. J. Biol. Macromol. 2018, 119, 453–461. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.M.; Luque de Castro, M.D. A review on enzyme and ultrasound: A controversial but fruitful relationship. Anal. Chim. Acta 2015, 889, 1–21. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Ultrasound assisted intensification of enzyme activity and its properties: A mini-review. World J. Microbiol. Biotechnol. 2017, 33, 1–12. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-E-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Paniwnyk, L. Applications of ultrasound in processing of liquid foods: A review. Ultrason. Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.E. Engineering Principles of Ultrasound Technology; Bermudez-Aguirre, D., Ed.; Academic Press: London, UK, 2017; ISBN 9780128046142. [Google Scholar]
- Bhangu, S.K.; Ashokkumar, M. Theory of Sonochemistry. Top. Curr. Chem. 2016, 374. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J. Sonochemistry: Theory, Appliations and Uses of Ultrasound in Chemistry; Ellis Horwood Publishers: New York, NY, USA, 1988; ISBN 0-7458-0240-0. [Google Scholar]
- Pierce, A.D. Mathematical theory of wave propagation. In Encyclopedia of Acoustics, Volume One; Crocker, M.J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; pp. 21–37. [Google Scholar]
- Blitz, J. Propagation of sound waves. In Elements of Acoustics; Butterworth & Co Publishers: London, UK, 1964; pp. 15–29. [Google Scholar]
- Colussi, A.J.; Weavers, L.; Hoffmann, K.R.M. Chemical Bubble Dynamics and Quantitative Sonochemistry. J. Phys. Chem. A 1998, 102, 6927–6934. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Kentish, S.E.; Stevens, G.W.; Ashokkumar, M. Application of ultrasound in membrane separation processes: A review. Rev. Chem. Eng. 2006, 22, 155–194. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Fu, N.; Zang, S. Hydrolysis of cellulose in 1-allyl-3-methylimidazolium chloride catalyzed by methyltrioxorhenium. Catal. Commun. 2016, 76, 46–49. [Google Scholar] [CrossRef]
- Mamvura, T.A.; Iyuke, S.E.; Paterson, A.E. Energy changes during use of high-power ultrasound on food grade surfaces. S. Afr. J. Chem. Eng. 2018, 25, 62–73. [Google Scholar] [CrossRef]
- Vinatoru, M. Ultrasonically assisted extraction (UAE) of natural products some guidelines for good practice and reporting. Ultrason. Sonochem. 2015, 25, 94–95. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.L.; Che Lah, N.F.; Ismail, S.; Ooi, B.S. Membrane antifouling methods and alternatives: Ultrasound approach. Sep. Purif. Rev. 2012, 41, 318–346. [Google Scholar] [CrossRef]
- Paniwnyk, L. Application of Ultrasound, 2nd ed; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780124114791. [Google Scholar]
- Peshkovsky, A.S. From Research to Production: Overcoming Scale-Up Limitations of Ultrasonic Processing. In Ultrasound: Advances in Food Processing and Preservation; Daniela Bermudez-Aguirre, Ed.; Academic Press: Boston, MA, USA, 2017; pp. 409–423. [Google Scholar]
- Xu, B.; Azam, S.M.R.; Feng, M.; Wu, B.; Yan, W.; Zhou, C.; Ma, H. Ultrasonics Sonochemistry Application of multi-frequency power ultrasound in selected food processing using large-scale reactors: A review. Ultrason. Sonochem. 2021, 81, 105855. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombout, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zeng, W.C.; Zhang, W.H.; Liao, X.P.; Shi, B. Effect of ultrasound on the activity and conformation of α-amylase, papain and pepsin. Ultrason. Sonochem. 2014, 21, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Prajapat, A.L.; Subhedar, P.B.; Gogate, P.R. Ultrasound assisted enzymatic depolymerization of aqueous guar gum solution. Ultrason. Sonochem. 2016, 29, 84–92. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Martin, G.J.O.; Ashokkumar, M. Mechanism of low-frequency and high-frequency ultrasound-induced inactivation of soy trypsin inhibitors. Food Chem. 2021, 360, 1–11. [Google Scholar] [CrossRef]
- O’Donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, W.; Zhang, L.; Tian, S.; Chen, F. Effect of ultrasound-assisted extraction on the structure and emulsifying properties of peanut protein isolate. J. Sci. Food Agric. 2021, 101, 1150–1160. [Google Scholar] [CrossRef]
- Galbán, J.; Andreu, Y.; Sierra, J.F.; De Marcos, S.; Castillo, J.R. Intrinsic fluorescence of enzymes and fluorescence of chemically modified enzymes for analytical purposes: A review. Luminescence 2001, 16, 199–210. [Google Scholar] [CrossRef]
- Abidi, M.; Iram, A.; Furkan, M.; Naeem, A. Secondary structural alterations in glucoamylase as an influence of protein aggregation. Int. J. Biol. Macromol. 2017, 98, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Almagro, N.; Montilla, A.; Moreno, F.J.; Villamiel, M. Modification of citrus and apple pectin by power ultrasound: Effects of acid and enzymatic treatment. Ultrason. Sonochem. 2017, 38, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Rico-Rodríguez, F.; Serrato, J.C.; Montilla, A.; Villamiel, M. Impact of ultrasound on galactooligosaccharides and gluconic acid production throughout a multienzymatic system. Ultrason. Sonochem. 2018, 44, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.B.; Gogate, P.R. Enhancing the activity of cellulase enzyme using ultrasonic irradiations. J. Mol. Catal. B Enzym. 2014, 101, 108–114. [Google Scholar] [CrossRef]
- Vanga, S.K.; Wang, J.; Raghavan, V. Effect of ultrasound and microwave processing on the structure, in-vitro digestibility and trypsin inhibitor activity of soymilk proteins. LWT 2020, 131, 109708. [Google Scholar] [CrossRef]
- Ma, H.; Huang, L.; Jia, J.; He, R.; Luo, L.; Zhu, W. Effect of energy-gathered ultrasound on Alcalase. Ultrason. Sonochem. 2011, 18, 419–424. [Google Scholar] [CrossRef]
- Bashari, M.; Eibaid, A.; Wang, J.; Tian, Y.; Xu, X.; Jin, Z. Influence of low ultrasound intensity on the degradation of dextran catalyzed by dextranase. Ultrason. Sonochem. 2013, 20, 155–161. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta - Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Şener, N.; Kiliç Apar, D.; Özbek, B. A modelling study on milk lactose hydrolysis and β-galactosidase stability under sonication. Process Biochem. 2006, 41, 1493–1500. [Google Scholar] [CrossRef]
- Mawson, R.; Gamage, M.; Terefe, N.S.; Knoerzer, K. Ultrasound in Enzyme Activation and Inactivation. In Ultrasound Technologies for Food and Bioprocessing. Food Engineering Series; Feng, H., Barbosa-Canovas, G.W.J., Eds.; Springer: New York, NY, USA, 2011; pp. 369–404. ISBN 978-1-4419-7471-6. [Google Scholar]
- Perino, S.; Chemat, F. Green process intensification techniques for bio-refinery. Curr. Opin. Food Sci. 2019, 25, 8–13. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Sui, X.; Zhang, Y.; Feng, H.; Jiang, L. Ultrasound-assisted aqueous enzymatic extraction of oil from perilla (Perilla frutescens L.) seeds Extracción enzimática acuosa asistida por ultrasonidos de aceite de perilla (Perilla frutescens L.). J. Food 2014, 12, 16–21. [Google Scholar]
- Rao, P.R.; Rathod, V.K. Mapping study of an ultrasonic bath for the extraction of andrographolide from Andrographis paniculata using ultrasound. Ind. Crops Prod. 2015, 66, 312–318. [Google Scholar] [CrossRef]
- Ke, L.Q. Optimization of Ultrasonic Extraction of Polysaccharides from Lentinus Edodes Based on Enzymatic Treatment. J. Food Process. Preserv. 2015, 39, 254–259. [Google Scholar] [CrossRef]
- Liao, N.; Zhong, J.; Ye, X.; Lu, S.; Wang, W.; Zhang, R.; Xu, J.; Chen, S.; Liu, D. Ultrasonic-assisted enzymatic extraction of polysaccharide from Corbicula fluminea: Characterization and antioxidant activity. LWT - Food Sci. Technol. 2015, 60, 1113–1121. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Tobiszewski, M.; Marć, M.; Gałuszka, A.; Namieśnik, J. Green Chemistry Metrics with Special Reference to Green Analytical Chemistry. Molecules 2015, 20, 10928–10946. [Google Scholar] [CrossRef]
- Haji Heidari, S.; Taghian Dinani, S. Ultrasound - Enzymatic Extraction of Peanut Oil. Eur. J. Lipid Sci. Technol. 2017, 120, 1700252. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Liu, Y.; Zhang, H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014, 150, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C.; Mo, Y. Effect of ultrasound on the activity of alliinase from fresh garlic. Ultrason. Sonochem. 2011, 18, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Tchabo, W.; Ma, Y.; Engmann, F.N.; Zhang, H. Ultrasound-assisted enzymatic extraction (UAEE) of phytochemical compounds from mulberry (Morus nigra) must and optimization study using response surface methodology. Ind. Crops Prod. 2015, 63, 214–225. [Google Scholar] [CrossRef]
- Bora, S.J.; Handique, J.; Sit, N. Effect of ultrasound and enzymatic pre-treatment on yield and properties of banana juice. Ultrason. Sonochem. 2017, 37, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Rigi, A.; Abbasi, S.; Scanlon, M.G. Enhanced lycopene extraction from tomato industrial waste using microemulsion technique: Optimization of enzymatic and ultrasound pre-treatments. Innov. Food Sci. Emerg. Technol. 2016, 35, 160–167. [Google Scholar] [CrossRef]
- Li, F.; Mao, Y.D.; Wang, Y.F.; Raza, A.; Qiu, L.P.; Xu, X.Q. Optimization of ultrasonic-assisted enzymatic extraction conditions for improving total phenolic content, antioxidant and antitumor activities in vitro from Trapa quadrispinosa Roxb. residues. Molecules 2017, 22, 396. [Google Scholar] [CrossRef]
- Goula, A.M.; Papatheodorou, A.; Karasavva, S.; Kaderides, K. Ultrasound-Assisted Aqueous Enzymatic Extraction of Oil from Pomegranate Seeds. Waste Biomass Valoriz. 2018, 9. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 0470174463. [Google Scholar]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Yang, Y.; Song, X.; Yu, Z. Optimization of ultrasound-assisted compound enzymatic extraction and characterization of polysaccharides from blackcurrant. Carbohydr. Polym. 2015, 117, 895–902. [Google Scholar] [CrossRef]
- Dabbour, M.; He, R.; Mintah, B.; Tang, Y.; Ma, H. Ultrasound assisted enzymolysis of sunflower meal protein: Kinetics and thermodynamics modeling. J. Food Process Eng. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Gao, X.; Feng, T.; Liu, E.; Shan, P.; Zhang, Z.; Liao, L.; Ma, H. Ougan juice debittering using ultrasound-aided enzymatic hydrolysis: Impacts on aroma and taste. Food Chem. 2021, 345, 128767. [Google Scholar] [CrossRef]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Demirhan, E.; Özbek, B. A modeling studyon hydrolysis of lactose recovered from whey and β-galactosidase stability under sonic treatment. Chem. Eng. Commun. 2009, 196, 767–787. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Q.; Bu, W.; Zhang, C.; Yang, Z.; Zhang, X.; Zhang, K. Ultrasound-assisted hydrolysis of lard for free fatty acids catalyzed by combined two lipases in aqueous medium. Bioengineered 2020, 11, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Dalagnol, L.M.G.; Silveira, V.C.C.; da Silva, H.B.; Manfroi, V.; Rodrigues, R.C. Improvement of pectinase, xylanase and cellulase activities by ultrasound: Effects on enzymes and substrates, kinetics and thermodynamic parameters. Process Biochem. 2017, 61, 80–87. [Google Scholar] [CrossRef]
- Wang, D.; Hou, F.; Ma, X.; Chen, W.; Yan, L.; Ding, T.; Ye, X.; Liu, D. Study on the mechanism of ultrasound-accelerated enzymatic hydrolysis of starch: Analysis of ultrasound effect on different objects. Int. J. Biol. Macromol. 2020, 148, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Li, D.; Zhu, C. The effect of ultrasound on the properties and conformation of glucoamylase. Int. J. Biol. Macromol. 2018, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Ma, H.; Wang, K.; Yagoub, A.E.G.A.; Owusu, J.; Qu, W.; He, R.; Zhou, C.; Ye, X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015, 24, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.; Ma, H.; Hayat, K.; Ren, X.; Ali, Z.; Duan, Y.; Rashid, M.T. Enzymolysis reaction kinetics and thermodynamics of rapeseed protein with sequential dual-frequency ultrasound pretreatment. Int. J. Food Sci. Technol. 2018, 53, 72–80. [Google Scholar] [CrossRef]
- Musa, A.; Ma, H.; Gasmalla, M.A.A.; Sarpong, F.; Awad, F.N.; Duan, Y. Effect of multi-frequency counter-current S type ultrasound pretreatment on the enzymatic hydrolysis of defatted corn germ protein: Kinetics and thermodynamics. Process Biochem. 2019, 87, 112–118. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, J.; Wang, L.; Lu, F.; Wei, B.; Azam, R.S.M.; Ren, X.; Zhou, C.; Ma, H.; Bhandari, B. Effect of multi-frequency power ultrasound (MFPU) treatment on enzyme hydrolysis of casein. Ultrason. Sonochem. 2020, 63, 104930. [Google Scholar] [CrossRef]
- Rufino, A.R.; Biaggio, F.C.; Santos, J.C.; de Castro, H.F. Screening of lipases for the synthesis of xylitol monoesters by chemoenzymatic esterification and the potential of microwave and ultrasound irradiations to enhance the reaction rate. Int. J. Biol. Macromol. 2010, 47, 5–9. [Google Scholar] [CrossRef]
- Tomke, P.D.; Zhao, X.; Chiplunkar, P.P.; Xu, B.; Wang, H.; Silva, C.; Rathod, V.K.; Cavaco-Paulo, A. Lipase-ultrasound assisted synthesis of polyesters. Ultrason. Sonochem. 2017, 38, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Galgali, A.; Gawas, S.D.; Rathod, V.K. Ultrasound assisted synthesis of citronellol laurate by using Novozym 435. Catal. Today 2018, 309, 133–139. [Google Scholar] [CrossRef]
- Waghmare, G.V.; Mudaliar, C.; Rathod, V.K. Optimization of the enzyme catalyzed ultrasound assisted synthesis of cinnamyl butyrate using response surface methodology. React. Kinet. Mech. Catal. 2020, 129, 421–441. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Tonetto, G.M. What Is The Importance of Structured Triglycerides and Diglycerides? In Enzymatic Synthesis of Structured Triglycerides. SpringerBriefs in Molecular Science; Ferreira, M.L., Tonetto, G.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–16. [Google Scholar]
- Liu, S.L.; Dong, X.Y.; Wei, F.; Wang, X.; Lv, X.; Zhong, J.; Wu, L.; Quek, S.Y.; Chen, H. Ultrasonic pretreatment in lipase-catalyzed synthesis of structured lipids with high 1,3-dioleoyl-2-palmitoylglycerol content. Ultrason. Sonochem. 2015, 23, 100–108. [Google Scholar] [CrossRef] [PubMed]
- More, S.B.; Gogate, P.R.; Waghmare, J.S.; Naik, S.N. Intensified synthesis of structured triacylglycerols from fish, flaxseed and rice bran oil using supercritical CO2 or ultrasound. Chem. Eng. Process. - Process Intensif. 2019, 144, 107650. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, L.; Huang, F.H.; Guo, P.M.; Wei, F.; Deng, Q.C.; Zheng, C.; Wan, C.Y. Ultrasound irradiation promoted lipase-catalyzed synthesis of flavonoid esters with unsaturated fatty acids. J. Mol. Catal. B Enzym. 2013, 95, 82–88. [Google Scholar] [CrossRef]
- Nieto, S.; Villa, R.; Donaire, A.; Lozano, P. Ultrasound-assisted enzymatic synthesis of xylitol fatty acid esters in solvent-free conditions. Ultrason. Sonochem. 2021, 75, 105606. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Vera, C.; Guerrero, C.; Illanes, A. Performance of an ultrafiltration membrane bioreactor (UF-MBR) as a processing strategy for the synthesis of galacto-oligosaccharides at high substrate concentrations. J. Biotechnol. 2016, 223, 26–35. [Google Scholar] [CrossRef]
- Vera, C.; Córdova, A.; Aburto, C.; Guerrero, C.; Suárez, S.; Illanes, A. Synthesis and purification of galacto-oligosaccharides: State of the art. World J. Microbiol. Biotechnol. 2016, 32. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Santibañez, L.; Cassano, A.; Ruby-Figueroa, R.; Illanes, A. Purification of galacto-oligosaccharides (GOS) by three-stage serial nanofiltration units under critical transmembrane pressure conditions. Chem. Eng. Res. Des. 2017, 117, 488–499. [Google Scholar] [CrossRef]
- Pal, P.; Kumar, R.; Banerjee, S. Manufacture of gluconic acid: A review towards process intensification for green production. Chem. Eng. Process. Process Intensif. 2016, 104, 160–171. [Google Scholar] [CrossRef]
- Rico-Rodríguez, F.; Villamiel, M.; Ruiz-Aceituno, L.; Serrato, J.C.; Montilla, A. Effect of the lactose source on the ultrasound-assisted enzymatic production of galactooligosaccharides and gluconic acid. Ultrason. Sonochem. 2020, 67, 104945. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Paniwnyk, L.; Herceg, Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014, 121, 15–23. [Google Scholar] [CrossRef]
- González-Delgado, I.; López-Muñoz, M.J.; Morales, G.; Segura, Y. Optimisation of the synthesis of high galacto-oligosaccharides (GOS) from lactose with β-galactosidase from Kluyveromyces lactis. Int. Dairy J. 2016, 61, 211–219. [Google Scholar] [CrossRef]




| Source | Biomolecule/Product | Enzyme | Extraction Conditions | Results | Reference |
|---|---|---|---|---|---|
| Lentinus edodes (Edible mushroom) | Polysaccharides | Cellulase | Water/mass of L. edodes was 30:1. Treatment was sequential: enzymatic treatment followed by ultrasound treatment. | The optimal conditions of ultrasonic extraction were: ultrasound power 340 W, and ultrasound time 14 min. Under these conditions, the yield of polysaccharides was 14.3% (w/w), (weight of polysaccharides/dry weight of L. edodes). | [66] |
| Corbicula lumine (Asian clam) | Polysaccharides | Papain | The volume of extraction was 50 mL, and the experiments were carried out applying ultrasound as pretreatment of the enzyme reaction. | The optimal extraction conditions in ultrasound power of 300 W were: temperature 62 °C and ultrasound time 32 min. The yield of polysaccharides was 36.8% (w/w), (weight of polysaccharides/weight of raw material). | [67] |
| Wheat bran | Polysaccharides | Xylanase | Working volume was 100 mL. Ultrasound was applied simultaneously with the enzyme. The process was carried out in an ultrasonic bath. | The optimum extraction conditions were: temperature 50 °C, 70 min, and ultrasonic power 180 W. Under these conditions, the experimental yield was 142.6 mg/g. | [71] |
| Curcubita moschata (Pumpkin) | Polysaccharides | Cellulase | Enzymatic extraction and ultrasound were simultaneous. Ultrasonic processing was carried out in a thermostatic ultrasonic processor. | The optimal conditions were: temperature 51.5 °C, ultrasonic power 440 W, and time 20 min. Under these conditions, the maximum yield was 4.33%. | [79] |
| Blackcurrant | Polysaccharides | Pectinase and papain | Blackcurrant fruits were processed simultaneously by ultrasound and enzymes. The fruits and enzymes were put into a 500 mL beaker, then aqueous solutions were added at different liquid to solid ratios (10:1–50:1 mL/g). The extraction process was carried out at 40 °C in an ultrasonic cell disintegrator. | The optimal conditions were: enzyme concentration 1.575%, temperature 40 °C, and time 25.6 min. Under these conditions, the yield of polysaccharides was 14.3% (w/w), (weight of polysaccharides/dry weight of sample). | [80] |
| Perilla frutescens seeds (Medicinal and edible plant of Asian origin) | Oil | Cellulase, Viscozyme L®, Alcalase 2.4L®, Protex 6L®, and Protex 7L® | Perilla seed kernel powder (50 g) was mixed with water at a ratio of 6:1 liquid/solid and treated by ultrasonic, thus totaling approximately 300 mL of extraction volume. Ultrasonic pretreatment was carried out on an ultrasonic homogenizer. | The optimum ultrasonic parameters were: 250 W of ultrasonic power, 30 min, and 50 °C. The highest oil yield was 81.74% and was achieved with cellulase. | [64] |
| Pomegranate seeds | Oil | Cellulase and Peclyve V | The extraction of pomegranate seed oil by enzymatic treatment was carried out simultaneously with ultrasound treatment. The sonication process was carried out using a probe-type ultrasonic, and the water/seeds ratios were varied between 2:1 and 6:1 mL/g. | Ultrasonic irradiation was applied at 130 W. The combined use of enzymes and ultrasound had a maximum oil recovery of 95.8% at extraction time of 10 min, using Peclyve V at 55 °C. | [77] |
| Peanut seeds | Oil | Cellulase | The ultrasound was used as pretreatment, the peanut seed powder (40 g) was mixed with n-hexane (160 mL) at a ratio of 1:4 solid/liquid. The sonication was carried out at the ultrasonic bath. | The ultrasound extraction process was applied at frequency of 250 Hz and at 45 °C. The optimum condition was ultrasonic pretreatment for 33.23 min and cellulase concentration of 1.47%. | [70] |
| Banana | Juice | Cellulase and pectinase | 100 g of the banana slices were mixed with distilled water to make pulp. The pulp was subjected to a pretreatment with ultrasonication in an ultrasound bath at 40 kHz. | Ultrasonic irradiation was applied at 50 W for 30 min. Ultrasound combined with both enzymes produced a maximum yield of 89.4% compared to 47.3% in the control. | [74] |
| Tomato residues | Lycopene | Endozym®-Pectofruit | The ultrasonic pretreatment was performed using a probe-type ultrasound. The extractions were performed in a double-walled cylindrical glass chamber (200 mL). | Combined sonication and enzymatic pretreatments improved the efficiency up to 39%, which was obtained in ultrasound treatment at 50 W for 30 s. | [75] |
| Morus nigra (Mulberry) | Flavonoids | Pectinex UF | The process was carried out with ultrasonic probe equipment. Mulberry must (300 g) was placed into an Erlenmeyer flask (500 mL) with the enzyme to be simultaneously sonicated. | The ultrasonic treatment was performed at 60 W, duty cycle (10 s on and 5 s off), at 20 °C. The UAEE treatment was employed to enhance the quality of the must and reduce the time during the maceration process of juice. | [73] |
| Trapa quadrispinosa residues (Water caltrop) | Phenolics | Cellulase | The stem powders (1 g) were placed into 100 mL Erlenmeyer flask. Extractions were carried out in an ultrasonic bath at 40 kHz. | The optimal UAEE conditions were 1.74% cellulase concentration, ultrasonic extraction time of 25.5 min, and temperature of 49 °C. The yield was 53.6 mg gallic acid equivalent/g dry weight. | [76] |
| Enzyme | Hydrolysis Conditions | Results | Reference |
|---|---|---|---|
| Lipases | Hydrolysis of lard catalyzed by 1,3-specific lipases from Rhizomucor miehei combined with a nonspecific mono and diacylglycerol lipase from Penicillium cyclopiumand assisted with ultrasound pretreatment for 5 min, frequency at 53 kHz and ultrasound power of 250 W. | When using combi-lipases, the hydrolysis degree was 78.1%. When combi-lipases were assisted with 5 min ultrasound pretreatment before the reaction, the hydrolysis degree reached 97%. | [66] |
| Pectinase, xylanase, and cellulase | The equipment used was an ultrasonic bath of 9.5 L of maximum capacity at ultrasonic frequency of 40 kHz and total ultrasonic power 220 W. | The results show that ultrasound treatment increased enzyme activities by 5% for pectinase, 30% for xylanase, and 25% for cellulase compared with mechanical stirring. The substrates were presonicated. | [67] |
| α-L-rhamnosidases, β-glucosidases, and limoninases | The sonication treatment was carried out at 40 kHz, 80 W/L, and 90 min. Working volume was ~300 mL. | The process of sonication significantly enhanced activities of α-L-rhamnosidases, β-glucosidases, and limoninases; also, the sonication reduced the hydrolysis time by 33% (30 min). | [71] |
| Glucoamylase | The ultrasound probe was inserted into a starch solution and glucoamylase solution. The sonication was carried out at different ultrasonic powers (45–360 W), temperatures (35–75 °C), and treatment times (10–50 min). Reaction volume was fixed at 25 mL. | Ultrasound produced a significant intensification of starch enzymatic hydrolysis catalyzed by glucoamylase; furthermore, the ultrasound promoted the enzymatic hydrolysis of amylopectin, significantly enhancing starch hydrolysis. | [79] |
| Glucoamylase | The glucoamylase solutions were subjected to different ultrasonic conditions of power (0, 420, 540 W) for 10 min at 60 °C. Ultrasonic reactor worked with 2 L. | The glucoamylase activity was increased by 21.07% over the control with ultrasound. However, at the application of high ultrasonic power (540 W), the rate of reaction decreased, probably due to decreased enzyme activity. | [80] |
| Alcalase | The ultrasound was used as pretreatment, using a probe at 200 W and five different frequencies (20, 28, 35, 40, 50 kHz). After pretreatments, the solutions were adjusted to temperature of 50 °C. Reaction vessel used was 600 mL. | The results showed that ultrasound pretreatment increased the degree of hydrolysis compared to that of the control for up to 75 min, even so, different substrate concentrations were used (5–25 g/L). | [64] |
| Alcalase | Ultrasound pretreatment for the enzyme hydrolysis of defatted corn germ protein with single frequency (20 KHz) and with a multi-frequency application (20, 28, 35, and 40 KHz) at constant Pd (100 W/L). Processing volume was ~1 L. | Ultrasound increased the reaction rate constant values in an average of 51%, while under the multi-frequency ultrasound scheme, it was increased by 56%. | [77] |
| Alcalase | The ultrasound substrate pretreatment with sweeping frequencies in cycles (40 +/− 2 kHz) and 200 was tested using a working solution of 300 mL. | Multi-frequency power ultrasound pretreatment was able to improve the enzymatic hydrolysis; kinetic studies showed that SFPU pretreatment decreased the apparent constant KM by 32.8%. | [70] |
| Alcalase | The experiments of multi-frequency power ultrasound pretreatments were conducted under different ultrasound frequency modes: mono-frequency (20, 40, and 60 kHz), dual-frequency (20/40, 20/60, and 40/60 kHz), and tri-frequency (20/40/60 kHz). The system had a volume capacity of 3 L. | Results showed that multi-frequency power ultrasound pretreatments in tri-frequency mode significantly improved the degree of hydrolysis value of casein in 12%. | [74] |
| β-galactosidase | Enzyme was sonicated at 20 kHz and acoustic power from 20 to 100 W, using milk as substrate in a reaction volume of 250 mL. | Ultrasonic treatment resulted in lactose hydrolysis degree of 90% and residual enzyme activity of 75% at the optimum operational conditions (acoustic power of 20 W, duty cycle rate of 10%, and enzyme concentration of 1 mL/L), resulting in a significant improvement compared to the control reaction without ultrasound. | [74] |
| Synthesized Product | US Reaction Conditions | Results | Reference |
|---|---|---|---|
| Synthesis by lipase of lipid structure with high content of 1,3-dioleoyl-2-palmitoylglycerol. | Lipozyme immobilized with an ultrasound pretreatment of 6 min, 50% power, 20 kHz, and 3 s on/9 s off duty cycle was applied. | With ultrasound, the OPO content increased to 35.9% in 1 h compared to 4 h without ultrasound. | [99] |
| Lipase synthesis of flavonoid esters with unsaturated fatty acids. | Novozym 435 was used, applying ultrasound pretreatment with a frequency of 25 kHz, power of 200 W for routine and 150 W for naringin for 1 h. | A conversion of 83.2% was obtained, reducing the reaction in 24 h as compared to the mechanical stirring. | [101] |
| Xylitol fatty acid esters. | Immobilized lipase B from Candida antarctica was used, applying a direct sonication pretreatment for 15 min (45 s/min pulses), different amplitudes from 10 to 100%, and 40 °C. | Up to 95% yield was achieved after 90 min at 40 °C. | [102] |
| Synthesis of structured triacylglycerols from fish, flaxseed, and rice bran oil. | Novozyme N-435 and Lipozyme RM (LRM) lipase were used, applying ultrasound probe at 22 kHz, 240 W, and testing different duty cycles. | 84.5% of product yield with an optimal cycle of 6 s/4 s (on/off) in 9.6 h. | [100] |
| Production of GOS and GA in a multi-enzyme system. | Commercial β-galactosidase from K. lactis and commercial glucose oxidase (Gox) from Aspergillus oryzae were used, applying sonifier at 20 kHz, 400 W, 30% amplitude, pulses of 3 s on/7 s off, and temperature between 40 °C and 45 °C. | The best GOS product composition of 49% and 28% GA was obtained after 2 h of reaction. | [54] |
| Production of GOS and GA in a multi-enzyme system with different sources of lactose. | Commercial β-galactosidase from K. lactis, commercial glucose oxidase (Gox) from Aspergillus oryzae were used, applying US at 20 kHz, 400 W, submerged at 2 cm depth from the surface of the liquid, amplitude of 0, 15, and 30%, duty cycle of 3 s on/7 s off, and the temperature was kept between 40 °C and 45 °C. | Maximum yield in the production of GOS 44.9% was obtained after 60 min of reaction and the production of GA depended on the intensity of ultrasound, achieving the highest amount of GA when the intensity was 30%. | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova, A.; Henríquez, P.; Nuñez, H.; Rico-Rodriguez, F.; Guerrero, C.; Astudillo-Castro, C.; Illanes, A. Recent Advances in the Application of Enzyme Processing Assisted by Ultrasound in Agri-Foods: A Review. Catalysts 2022, 12, 107. https://doi.org/10.3390/catal12010107
Córdova A, Henríquez P, Nuñez H, Rico-Rodriguez F, Guerrero C, Astudillo-Castro C, Illanes A. Recent Advances in the Application of Enzyme Processing Assisted by Ultrasound in Agri-Foods: A Review. Catalysts. 2022; 12(1):107. https://doi.org/10.3390/catal12010107
Chicago/Turabian StyleCórdova, Andrés, Paola Henríquez, Helena Nuñez, Fabián Rico-Rodriguez, Cecilia Guerrero, Carolina Astudillo-Castro, and Andrés Illanes. 2022. "Recent Advances in the Application of Enzyme Processing Assisted by Ultrasound in Agri-Foods: A Review" Catalysts 12, no. 1: 107. https://doi.org/10.3390/catal12010107
APA StyleCórdova, A., Henríquez, P., Nuñez, H., Rico-Rodriguez, F., Guerrero, C., Astudillo-Castro, C., & Illanes, A. (2022). Recent Advances in the Application of Enzyme Processing Assisted by Ultrasound in Agri-Foods: A Review. Catalysts, 12(1), 107. https://doi.org/10.3390/catal12010107