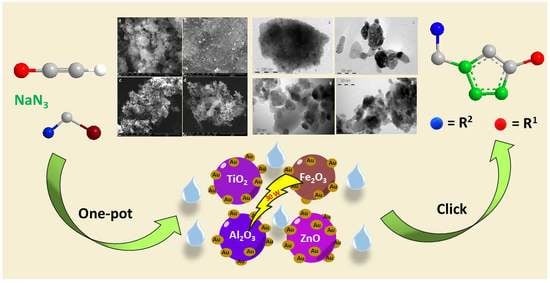
Heterogeneous Gold Nanoparticle-Based Catalysts for the Synthesis of Click-Derived Triazoles via the Azide-Alkyne Cycloaddition Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterisation of Supported Au NPs
2.2. Synthesis of 1,2,3-Triazoles Using Au NPs on Different Supports
3. Experimental
3.1. Materials
3.2. Deposition of Au NPs
3.3. Characterization of the Supported Au Nanoparticles
3.4. Gold Nanoparticle-Catalyzed Azide-Alkyne Cycloaddition Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chng, L.L.; Erathodiyil, N.; Ying, J.Y. Nanostructured catalysts for organic transformations. Acc. Chem. Res. 2013, 46, 1825–1837. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Bimetallic nanocrystals: Liquid-phase synthesis and catalytic applications. Adv. Mater. 2011, 23, 1044–1060. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Len, C.; Fihri, A. Silica-supported palladium: Sustainable catalysts for cross-coupling reactions. Coord. Chem. Rev. 2009, 253, 2599–2626. [Google Scholar] [CrossRef]
- Boominathan, M.; Pugazhenthiran, N.; Nagaraj, M.; Muthusubramanian, S.; Murugesan, S.; Bhuvanesh, N. Nanoporous titania-supported gold nanoparticle-catalyzed green synthesis of 1,2,3-triazoles in aqueous medium. ACS Sustain. Chem. Eng. 2013, 1, 1405–1411. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Wang, J.; Rocha, B.G.M.; Maldonado-Hódar, F.J.; Pombeiro, A.J.L.O. Supported Gold Nanoparticles as Reusable Catalysts for Oxidation Reactions of Industrial Significance. ChemCatChem 2017, 9, 1211–1221. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–775. [Google Scholar] [CrossRef]
- Yan, N.; Xiao, C.; Kou, Y. Transition metal nanoparticle catalysis in green solvents. Coord. Chem. Rev. 2010, 254, 1179–1218. [Google Scholar] [CrossRef]
- deAlmeida, M.P.; Carabineiro, S.A.C. The Best of Two Worlds from the Gold Catalysis Universe: Making Homogeneous Heterogeneous. ChemCatChem 2012, 4, 18–29. [Google Scholar] [CrossRef]
- Hashmi, A.S.K. Homogeneous catalysis by gold. Gold Bull. 2004, 37, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef]
- Hughes, M.D.; Xu, Y.J.; Jenkins, P.; McMorn, P.; Landon, P.; Enache, D.I.; Carley, A.F.; Attard, G.A.; Hutchings, G.J.; King, F.; et al. Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions. Nature 2005, 437, 1132–1135. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Bujak, P.; Bartczak, P.; Polanski, J. Highly efficient room-temperature oxidation of cyclohexene and d-glucose over nanogold Au/SiO2 in water. J. Catal. 2012, 295, 15–21. [Google Scholar] [CrossRef]
- Jin, Z.; Song, Y.Y.; Fu, X.P.; Song, Q.S.; Jia, C.J. Nanoceria Supported Gold Catalysts for CO Oxidation. Chin. J. Chem. 2018, 36, 639–643. [Google Scholar] [CrossRef]
- Carrettin, S.; Blanco, M.C.; Corma, A.; Hashmi, A.S.K. Heterogeneous gold-catalysed synthesis of phenols. Adv. Synth. Catal. 2006, 348, 1283–1288. [Google Scholar] [CrossRef]
- Corma, A.; Leyva-Pérez, A.; Sabater, M.J. Gold-Catalyzed Carbon–Heteroatom Bond-Forming Reactions. Chem. Rev. 2011, 111, 1657–1712. [Google Scholar] [CrossRef]
- Stephen, A.; Hashmi, K. Homogeneous gold catalysis beyond assumptions and proposals-characterized intermediates. Angew. Chem. Int. Ed. 2010, 49, 5232–5241. [Google Scholar] [CrossRef]
- Rej, S.; Chanda, K.; Chiu, C.-Y.; Huang, M.H. Control of Regioselectivity over Gold Nanocrystals of Different Surfaces for the Synthesis of 1,4-Disubstituted Triazole through the Click Reaction. Chem.-Eur. J. 2014, 20, 15991–15997. [Google Scholar] [CrossRef]
- Díaz Arado, O.; Mönig, H.; Wagner, H.; Franke, J.H.; Langewisch, G.; Held, P.A.; Studer, A.; Fuchs, H. On-surface azide-alkyne cycloaddition on Au(111). ACS Nano 2013, 7, 8509–8515. [Google Scholar] [CrossRef] [PubMed]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem.-Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- Tullis, J.S.; VanRens, J.C.; Natchus, M.G.; Clark, M.P.; De, B.; Hsieh, L.C.; Janusz, M.J. The development of new triazole based inhibitors of tumor necrosis factor-α (TNF-α) production. Bioorg. Med. Chem. Lett. 2003, 13, 1665–1668. [Google Scholar] [CrossRef]
- Chu, C.; Liu, R. Application of click chemistry on preparation of separation materials for liquid chromatography. Chem. Soc. Rev. 2011, 40, 2177–2188. [Google Scholar] [CrossRef]
- Lau, Y.H.; Rutledge, P.J.; Watkinson, M.; Todd, M.H. Chemical sensors that incorporate click-derived triazoles. Chem. Soc. Rev. 2011, 40, 2848–2866. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Guedes Da Silva, M.F.C.; Sokolnicki, J.; Smoleński, P.; Pombeiro, A.J.L. Hydrosoluble Cu(I)-DAPTA complexes: Synthesis, characterization, luminescence thermochromism and catalytic activity for microwave-assisted three-component azide-alkyne cycloaddition click reaction. Dalt. Trans. 2018, 47, 7290–7299. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.G.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Copper complexes bearing C-scorpionate ligands: Synthesis, characterization and catalytic activity for azide-alkyne cycloaddition in aqueous medium. Inorg. Chim. Acta 2018, 483, 371–378. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Guedes Da Silva, M.F.C.; Mahmudov, K.T.; Pombeiro, A.J.L. Arylhydrazone ligands as Cu-protectors and -catalysis promoters in the azide-alkyne cycloaddition reaction. Dalt. Trans. 2019, 48, 1774–1785. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Smolénski, P.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. Water-Soluble O-, S-and Se-Functionalized Cyclic Acetyl-triaza-phosphines. Synthesis, Characterization and Application in Catalytic Azide-alkyne Cycloaddition. Molecules 2020, 25, 5479. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. A new amido-phosphane as ligand for copper and silver complexes. Synthesis, characterization and catalytic application for azide–alkyne cycloaddition in glycerol. Dalt. Trans. 2021, 50, 6109–6125. [Google Scholar] [CrossRef] [PubMed]
- Librando, I.L.; Mahmoud, A.G.; Carabineiro, S.A.C.; Guedes Da Silva, M.F.C.; Geraldes, C.F.G.C.; Pombeiro, A.J.L. The catalytic activity of carbon-supported Cu(I)-phosphine complexes for the microwave-assisted synthesis of 1,2,3-triazoles. Catalysts 2021, 11, 185. [Google Scholar] [CrossRef]
- Librando, I.L.; Mahmoud, A.G.; Carabineiro, S.A.C.; Guedes da Silva, M.F.C.; Geraldes, C.F.G.C.; Pombeiro, A.J.L. Synthesis of a novel series of Cu(I) complexes bearing alkylated 1,3,5-triaza-7-phosphaadamantane as homogeneous and carbon-supported catalysts for the synthesis of 1-and 2-substituted-1,2,3-triazoles. Nanomaterials 2021, 11, 2702. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.S.; Jardim, G.A.M.; de Carvalho, R.L.; Araujo, M.H.; da Silva Júnior, E.N. Beyond copper-catalyzed azide-alkyne 1,3-dipolar cycloaddition: Synthesis and mechanism insights. Tetrahedron 2019, 75, 3697–3712. [Google Scholar] [CrossRef]
- Kalra, P.; Kaur, R.; Singh, G.; Singh, H.; Singh, G.; Pawan; Kaur, G.; Singh, J. Metals as “Click” catalysts for alkyne-azide cycloaddition reactions: An overview. J. Organomet. Chem. 2021, 944, 121846. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Speers, A.E.; Adam, G.C.; Cravatt, B.F. Activity-based protein profiling in vivo using a copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 4686–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, W.; Kluger, C. Azide/Alkyne- “Click” Reactions: Applications in Material Science and Organic Synthesis. Curr. Org. Chem. 2006, 10, 1791–1815. [Google Scholar] [CrossRef]
- Lutz, J.F. 1,3-Dipolar cycloadditions of azides and alkynes: A universal ligation tool in polymer and materials science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Copper nanoparticles in click chemistry: An alternative catalytic system for the cycloaddition of terminal alkynes and azides. Tetrahedron Lett. 2009, 50, 2358–2362. [Google Scholar] [CrossRef]
- Mularski, J.; Czaplińska, B.; Cieślik, W.; Bebłot, J.; Bartczak, P.; Sitko, R.; Polański, J.; Musiol, R. Electrolytic copper as cheap and effective catalyst for one-pot triazole synthesis. Sci. Rep. 2018, 8, 4496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Gold Council. Available online: https://www.gold.org/news-and-events/press-releases/gold-reference-catalysts-now-available-international-standard (accessed on 25 June 2021).
- Carabineiro, S.A.C.; Tavares, P.B.; Figueiredo, J.L. Gold on oxide-doped alumina supports as catalysts for CO oxidation. Appl. Nanosci. 2012, 2, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Milone, C.; Crisafulli, C.; Ingoglia, R.; Schipilliti, L.; Galvagno, S. A comparative study on the selective hydrogenation of α,β unsaturated aldehyde and ketone to unsaturated alcohols on Au supported catalysts. Catal. Today 2007, 122, 341–351. [Google Scholar] [CrossRef]
- Albonetti, S.; Bonelli, R.; Delaigle, R.; Femoni, C.; Gaigneaux, E.M.; Morandi, V.; Ortolani, L.; Tiozzo, C.; Zacchini, S.; Trifirò, F. Catalytic combustion of toluene over cluster-derived gold/iron catalysts. Appl. Catal. A Gen. 2010, 372, 138–146. [Google Scholar] [CrossRef]
- Solsona, B.E.; Garcia, T.; Jones, C.; Taylor, S.H.; Carley, A.F.; Hutchings, G.J. Supported gold catalysts for the total oxidation of alkanes and carbon monoxide. Appl. Catal. A Gen. 2006, 312, 67–76. [Google Scholar] [CrossRef]
- Hua, J.; Wei, K.; Zheng, Q.; Lin, X. Influence of calcination temperature on the structure and catalytic performance of Au/iron oxide catalysts for water-gas shift reaction. Appl. Catal. A Gen. 2004, 259, 121–130. [Google Scholar] [CrossRef]
- Neri, G.; Visco, A.M.; Galvagno, S.; Donato, A.; Panzalorto, M. Au/iron oxide catalysts: Temperature programmed reduction and X-ray diffraction characterization. Thermochim. Acta 1999, 329, 39–46. [Google Scholar] [CrossRef]
- PalDey, S.; Gedevanishvili, S.; Zhang, W.; Rasouli, F. Evaluation of a spinel based pigment system as a CO oxidation catalyst. Appl. Catal. B Environ. 2005, 56, 241–250. [Google Scholar] [CrossRef]
- Khoudiakov, M.; Gupta, M.C.; Deevi, S. Au/Fe2O3 nanocatalysts for CO oxidation: A comparative study of deposition-precipitation and coprecipitation techniques. Appl. Catal. A Gen. 2005, 291, 151–161. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Tanaka, K. ichi Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, K.Q.; Yu, J.; Xu, B.Q. A key to the storage stability of Au/TiO2 catalyst. Phys. Chem. Chem. Phys. 2008, 10, 6399–6404. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Kang, W.; Xie, K. Comparison of reduction behavior of Fe2O3, ZnO and ZnFe2O4 by TPR technique. J. Nat. Gas Chem. 2009, 18, 110–113. [Google Scholar] [CrossRef]
- Valenzuela, M.A.; Bosch, P.; Jiménez-Becerrill, J.; Quiroz, O.; Páez, A.I. Preparation, characterization and photocatalytic activityof ZnO, Fe2O3 and ZnFe2O4. J. Photochem. Photobiol. A Chem. 2002, 148, 177–182. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Machado, B.F.; Bacsa, R.R.; Serp, P.; Draić, G.; Faria, J.L.; Figueiredo, J.L. Catalytic performance of Au/ZnO nanocatalysts for CO oxidation. J. Catal. 2010, 273, 191–198. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Bogdanchikova, N.; Tavares, P.B.; Figueiredo, J.L. Nanostructured iron oxide catalysts with gold for the oxidation of carbon monoxide. RSC Adv. 2012, 2, 2957–2965. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Carabineiro, S.A.C.; Maldonado-Hódar, F.J.; Madeira, L.M. Wet peroxide oxidation of dye-containing wastewaters using nanosized Au supported on Al2O3. Catal. Today 2017, 280, 165–175. [Google Scholar] [CrossRef]
- Rodrigues, C.S.D.; Carabineiro, S.A.C.; Maldonado-Hódar, F.J.; Madeira, L.M. Orange II Degradation by Wet Peroxide Oxidation Using Au Nanosized Catalysts: Effect of the Support. Ind. Eng. Chem. Res. 2017, 56, 1988–1998. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-dependent catalytic activity and dynamics of gold nanoparticles at the single-molecule level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, G.; Sun, J.; Cao, X.; He, Y.; Feng, J.; Li, D. The effect of oxygen vacancies in ZnO at an Au/ZnO interface on its catalytic selective oxidation of glycerol. J. Catal. 2019, 377, 271–282. [Google Scholar] [CrossRef]
- Strunk, J.; Kähler, K.; Xia, X.; Comotti, M.; Schüth, F.; Reinecke, T.; Muhler, M. Au/ZnO as catalyst for methanol synthesis: The role of oxygen vacancies. Appl. Catal. A Gen. 2009, 359, 121–128. [Google Scholar] [CrossRef]
- Polarz, S.; Strunk, J.; Ischenko, V.; Van Den Berg, M.W.E.; Hinrichsen, O.; Muhler, M.; Driess, M. On the role of oxygen defects in the catalytic performance of zinc oxide. Angew. Chem. Int. Ed. 2006, 45, 2965–2969. [Google Scholar] [CrossRef] [Green Version]
- Park, S.M.; Ikegami, T.; Ebihara, K. Effects of substrate temperature on the properties of Ga-doped ZnO by pulsed laser deposition. Thin Solid Films 2006, 513, 90–94. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Yu, Y.H.; Pei, Z.L.; Bai, X.D.; Sun, C.; Huang, R.F.; Wen, L.S. X-ray photoelectron spectroscopy and auger electron spectroscopy studies of Al-doped ZO films. Appl. Surf. Sci. 2000, 158, 134–140. [Google Scholar] [CrossRef]
- Kidwai, M.; Bansal, V.; Kumar, A.; Mozumdar, S. The first Au-nanoparticles catalyzed green synthesis of propargylamines via a three-component coupling reaction of aldehyde, alkyne and amine. Green Chem. 2007, 9, 742–774. [Google Scholar] [CrossRef]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed azide-alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef] [Green Version]
- Maity, P.; Takano, S.; Yamazoe, S.; Wakabayashi, T.; Tsukuda, T. Binding motif of terminal alkynes on gold clusters. J. Am. Chem. Soc. 2013, 135, 9450–9457. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Carabineiro, S.A.C.; Bakker, J.J.W.; Soares, O.S.G.P.; Chen, X.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L.; Gascon, J.; Kapteijn, F. Stabilized gold on cerium-modified cryptomelane: Highly active in low-temperature CO oxidation. J. Catal. 2014, 309, 58–65. [Google Scholar] [CrossRef]









| Sample | SBET, m2 g−1 a | Total Pore Volume, cm3 g−1 | Pore Size, nm | Phase Detected b | TPR Peaks, °C a |
|---|---|---|---|---|---|
| Al2O3 | 210 | 1.24 | 19.6 | θ alumina; γ-alumina | 530, 550 * |
| Au/Al2O3 | 210 | n.d | n.d | n.d | 500, 810 * |
| Fe2O3 | 6 | 0.62 | 3.1 | hematite, α-Fe2O3 | 245, 391, 660, 896 |
| Au/Fe2O3 | 5 | n.d | n.d | hematite, α-Fe2O3; gold not detected | 75, 274, 350, 599, 701, 879 |
| TiO2 | 51 | 0.25 | 3.4 | Anatase (80%), rutile (20%) | 400, 438 * |
| Au/TiO2 | 49 | n.d | n.d | n.d | 168, 240, 371 *, 575 * |
| ZnO | 26 | 0.08 | 12.5 | ZnO | 376, 436 *, 827 |
| Au/ZnO | 25 | n.d | n.d | n.d | 452, 595, 941 |
| Au Material | Au Material | ||||
|---|---|---|---|---|---|
| Size Range, nm | Average Particle Size, nm | Oxidation State | Loading, wt% | Dispersion,% c | |
| Au/Al2O3 | 1–20 | 3.6 | Au0 | 0.7 | 32 |
| Au/Fe2O3 | 1–7 | 2.3 | Au+ | 0.8 | 50 |
| Au/TiO2 | 1–12 | 2.2 | Au+ | 1.6 | 53 |
| Au/ TiO2 (W) | n.a. | 3.7 b | n.a. | 1.5 | 31 |
| Au/ZnO | 1–10 | 5.5 | Au0 | 1.2 | 21 |
| Au/C (W) | n.a. | 10.5 b | n.a. | 1.0 | 11 |
| Entry | Catalyst | Catalyst Loading, b mol% | Temperature °C | Yield c % | TON d |
|---|---|---|---|---|---|
| 1 | Au/ZnO | 0.1 | 100 | 28 | 279 |
| 2 | Au/Fe2O3 | 0.1 | 100 | 41 | 414 |
| 3 | Au/TiO2 | 0.1 | 100 | 40 | 395 |
| 4 | Au/Al2O3 | 0.1 | 100 | 43 | 431 |
| 5 | Au/TiO2 (W) | 0.1 | 100 | 28 | 291 |
| 6 | Au/C (W) | 0.1 | 100 | 27 | 269 |
| 7 | Au/ZnO | 0.1 | 150 | 63 | 633 |
| 8 | Au/Fe2O3 | 0.1 | 150 | 66 | 659 |
| 9 | Au/TiO2 | 0.1 | 150 | 64 | 639 |
| 10 | Au/Al2O3 | 0.1 | 150 | 67 | 672 |
| 11 | Au/TiO2 (W) | 0.1 | 150 | 60 | 602 |
| 12 | Au/C (W) | 0.1 | 150 | 62 | 622 |
| 13 | Blank | - | 150 | 28 | n/a |
| 14 | Au/ZnO | 0.5 | 150 | 67 | 134 |
| 15 | Au/Fe2O3 | 0.5 | 150 | 73 | 146 |
| 16 | Au/TiO2 | 0.5 | 150 | 75 | 150 |
| 17 | Au/Al2O3 | 0.5 | 150 | 70 | 140 |
| 18 | Au/TiO2 (W) | 0.5 | 150 | 69 | 139 |
| 19 | Au/C (W) | 0.5 | 150 | 67 | 134 |
| 20 | Au/TiO2 | 1.0 | 150 | 79 | 79 |
| 21 | Au/TiO2 | 1.5 | 150 | 76 | 51 |
| 22 | Au/TiO2 e | 0.5 | 150 | 74 | 149 |
| 23 | Au/TiO2 f | 0.5 | 150 | 73 | 146 |
| Entry | Benzyl Bromide | Alkyne | Product | Yield, b % | TON, c |
|---|---|---|---|---|---|
| 1 |  |  |  | 79 | 79 |
| 2 |  |  |  | 62 | 62 |
| 3 |  |  |  | 74 | 74 |
| 4 |  |  |  | 71 | 71 |
| 5 |  |  |  | 75 | 75 |
| 6 |  |  |  | 46 | 46 |
| 7 |  |  |  | 39 | 39 |
| 8 |  |  |  | 71 | 71 |
| 9 |  |  |  | 63 | 63 |
| 10 |  |  |  | 64 | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Librando, I.L.; Mahmoud, A.G.; Carabineiro, S.A.C.; Guedes da Silva, M.F.C.; Maldonado-Hódar, F.J.; Geraldes, C.F.G.C.; Pombeiro, A.J.L. Heterogeneous Gold Nanoparticle-Based Catalysts for the Synthesis of Click-Derived Triazoles via the Azide-Alkyne Cycloaddition Reaction. Catalysts 2022, 12, 45. https://doi.org/10.3390/catal12010045
Librando IL, Mahmoud AG, Carabineiro SAC, Guedes da Silva MFC, Maldonado-Hódar FJ, Geraldes CFGC, Pombeiro AJL. Heterogeneous Gold Nanoparticle-Based Catalysts for the Synthesis of Click-Derived Triazoles via the Azide-Alkyne Cycloaddition Reaction. Catalysts. 2022; 12(1):45. https://doi.org/10.3390/catal12010045
Chicago/Turabian StyleLibrando, Ivy L., Abdallah G. Mahmoud, Sónia A. C. Carabineiro, M. Fátima C. Guedes da Silva, Francisco J. Maldonado-Hódar, Carlos F. G. C. Geraldes, and Armando J. L. Pombeiro. 2022. "Heterogeneous Gold Nanoparticle-Based Catalysts for the Synthesis of Click-Derived Triazoles via the Azide-Alkyne Cycloaddition Reaction" Catalysts 12, no. 1: 45. https://doi.org/10.3390/catal12010045
APA StyleLibrando, I. L., Mahmoud, A. G., Carabineiro, S. A. C., Guedes da Silva, M. F. C., Maldonado-Hódar, F. J., Geraldes, C. F. G. C., & Pombeiro, A. J. L. (2022). Heterogeneous Gold Nanoparticle-Based Catalysts for the Synthesis of Click-Derived Triazoles via the Azide-Alkyne Cycloaddition Reaction. Catalysts, 12(1), 45. https://doi.org/10.3390/catal12010045