Mixed Metal Oxides of M1 MoVNbTeOx and TiO2 as Composite Catalyst for Oxidative Dehydrogenation of Ethane
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Catalysts
2.2. Characterization of Catalysts
2.3. Evaluation of the Catalysts
3. Results and Discussion
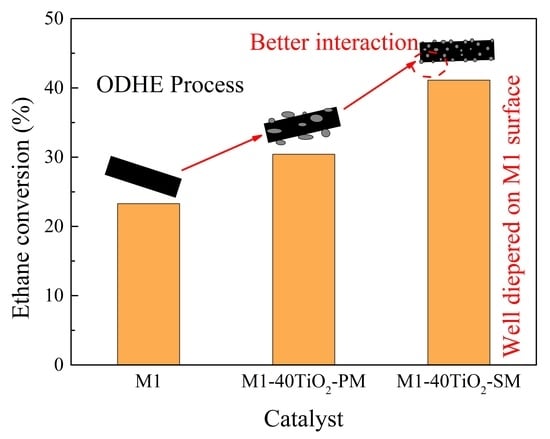
3.1. Reaction Performance in the ODHE Process
3.1.1. Catalytic Performance
3.1.2. Catalyst Stability
3.2. Catalyst Characterization
3.2.1. Elemental Compositions and Textural Properties
3.2.2. XRD
3.2.3. Catalysts Morphologies
3.2.4. XPS Analysis
3.2.5. H2-TPR and C2H6-TPSR Characterizations
3.2.6. Raman Spectra
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gartner, C.A.; van Veen, A.C.; Lercher, J.A. Oxidative Dehydrogenation of Ethane: Common Principles and Mechanistic Aspects. ChemCatChem 2013, 5, 3196–3217. [Google Scholar] [CrossRef]
- Najari, S.; Saeidi, S.; Concepcion, P.; Dionysiou, D.D.; Bhargava, S.K.; Lee, A.F.; Wilson, K. Oxidative dehydrogenation of ethane: Catalytic and mechanistic aspects and future trends. Chem. Soc. Rev. 2021, 50, 4564–4605. [Google Scholar] [CrossRef]
- Zhu, H.D.C.; Rosenfeld, M.; Harb, D.H.; Anjum, M.N.; Hedhili, S.; Ould-Chikh, J.-M. Basset. Ni–M–O (M = Sn, Ti, W) Catalysts Prepared by a Dry Mixing Method for Oxidative Dehydrogenation of Ethane. ACS Catal. 2016, 6, 2852–2866. [Google Scholar] [CrossRef] [Green Version]
- Solsona, B.; Ivars, F.; Dejoz, A.; Concepcion, P.; Vázquez, M.I.; Nieto, J.M.L. Supported Ni–W–O Mixed Oxides as Selective Catalysts for the Oxidative Dehydrogenation of Ethane. Top. Catal. 2009, 52, 751–757. [Google Scholar] [CrossRef]
- Botella, P.; Garcia-Gonzalez, E.; Dejoz, A.; Nieto, J.M.L.; Vazquez, M.I.; Gonzalez-Calbet, J. Selective Oxidative Dehydrogenation of Ethane on MoVTeNbO Mixed Metal Oxide Catalysts. J. Catal. 2004, 225, 428–438. [Google Scholar] [CrossRef]
- Nieto, L.; José, M. The Selective Oxidative Activation of Light Alkanes. From Supported Vanadia to Multicomponent Bulk V-Containing Catalysts. Top. Catal. 2006, 41, 3–15. [Google Scholar] [CrossRef]
- Botella, P.; Dejoz, A.; Nieto, J.L.; Concepción, P.; Vázquez, M. Selective oxidative dehydrogenation of ethane over MoVSbO mixed oxide catalysts. Appl. Catal. A Gen. 2006, 298, 16–23. [Google Scholar] [CrossRef]
- Heracleous, E.; Lemonidou, A. Ni–Nb–O mixed oxides as highly active and selective catalysts for ethene production via ethane oxidative dehydrogenation. Part II: Mechanistic aspects and kinetic modeling. J. Catal. 2006, 237, 175–189. [Google Scholar] [CrossRef]
- Chu, B.; An, H.; Nijhuis, T.; Schouten, J.C.; Cheng, Y. A self-redox pure-phase M1 MoVNbTeO/CeO2 nanocomposite as a highly active catalyst for oxidative dehydrogenation of ethane. J. Catal. 2015, 329, 471–478. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Aouine, M.; Millet, J.M.M. Optimizing the Efficiency of MoVNbTeO Catalysts for Ethane Oxidative Dehydrogenation to Ethylene. Catal. Commun. 2012, 21, 22–26. [Google Scholar] [CrossRef]
- Nieto, J.M.L.; Solsona, B.; Grasselli, R.K.; Concepción, P. Promoted NiO Catalysts for the Oxidative Dehydrogenation of Ethane. Top. Catal. 2014, 57, 1248–1255. [Google Scholar] [CrossRef]
- Popescu, I.; Heracleous, E.; Skoufa, Z.; Lemonidou, A.; Marcu, I.-C. Study by electrical conductivity measurements of semiconductive and redox properties of M-doped NiO (M = Li, Mg, Al, Ga, Ti, Nb) catalysts for the oxidative dehydrogenation of ethane. Phys. Chem. Chem. Phys. 2014, 16, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Wang, F.; Xu, G.Q. Tuning surface V5+ concentration in M1 phase MoVSbOx catalysts for ethylene production from ethane through oxidative dehydrogenation reaction. Appl. Catal. A Gen. 2021, 610, 117946. [Google Scholar] [CrossRef]
- Gao, Y.; Neal, L.M.; Li, F. Li-Promoted LaxSr2–XFeO4−δ Core–Shell Redox Catalysts for Oxidative Dehydrogenation of Ethane under a Cyclic Redox Scheme. ACS Catal. 2016, 6, 7293–7302. [Google Scholar] [CrossRef]
- Baca, M.; Millet, J.M.M. Bulk Oxidation State of the Different Cationic Elements in the MoVTe(Sb)NbO Catalysts for Oxidation or Ammoxidation of Propane. Appl. Catal. A Gen. 2005, 279, 67–77. [Google Scholar] [CrossRef]
- Ishikawa, S.; Yi, X.; Murayama, T.; Ueda, W. Heptagonal channel micropore of orthorhombic Mo3VOx as catalysis field for the selective oxidation of ethane. Appl. Catal. A Gen. 2014, 474, 10–17. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Ding, J.; Chai, R.J.; Zhao, G.F.; Liu, Y.; Lu, Y. Oxidative Dehydrogenation of Ethane to Ethylene: A Promising CeO2-ZrO2-Modified NiO-Al2O3/Ni-Foam Catalyst. Appl. Catal. A Gen. 2018, 550, 151–159. [Google Scholar] [CrossRef]
- Bortolozzi, J.; Banús, E.; Terzaghi, D.; Gutierrez, L.; Milt, V.; Ulla, M. Novel catalytic ceramic papers applied to oxidative dehydrogenation of ethane. Catal. Today 2013, 216, 24–29. [Google Scholar] [CrossRef]
- Melzer, D.; Mestl, G.; Wanninger, K.; Zhu, Y.; Browning, N.D.; Sanchez-Sanchez, M.; Lercher, J.A. Design and Synthesis of Highly Active Movtenb-Oxides for Ethane Oxidative Dehydrogenation. Nat. Commun. 2019, 10, 4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.; Zhao, K.; Jiang, S.; Zhao, Z.; Cao, Y.; He, F. Alkali-metal enhanced LaMnO3 perovskite oxides for chemical looping oxidative dehydrogenation of ethane. Appl. Catal. A Gen. 2021, 609, 117910. [Google Scholar] [CrossRef]
- Zhu, H.; Ould-Chikh, S.; Anjum, D.H.; Sun, M.; Biausque, G.; Basset, J.M.; Caps, V. Nb effect in the nickel oxide-catalyzed low-temperature oxidative dehydrogenation of ethane. J. Catal. 2012, 285, 292–303. [Google Scholar] [CrossRef]
- Delgado, D.; Sanchis, R.; Cecilia, J.A.; Rodriguez-Castellon, E.; Caballero, A.; Solsona, B.; Nieto, J.M.L. Support Effects on Nio-Based Catalysts for the Oxidative Dehydrogenation (ODH) of Ethane. Catal. Today 2019, 333, 10–16. [Google Scholar] [CrossRef]
- Wang, C.; Yang, B.; Gu, Q.; Han, Y.; Tian, M.; Su, Y.; Pan, X.; Kang, Y.; Huang, C.; Liu, H.; et al. Near 100% ethene selectivity achieved by tailoring dual active sites to isolate dehydrogenation and oxidation. Nat. Commun. 2021, 12, 5447. [Google Scholar] [CrossRef]
- Ishchenko, E.; Kardash, T.; Gulyaev, R.; Sobolev, V.; Bondareva, V. Effect of K and Bi doping on the M1 phase in MoVTeNbO catalysts for ethane oxidative conversion to ethylene. Appl. Catal. A Gen. 2016, 514, 1–13. [Google Scholar] [CrossRef]
- Watanabe, H.; Koyasu, Y. New Synthesis Route for Mo–V–Nb–Te Mixed Oxides Catalyst for Propane Ammoxidation. Appl. Catal. A Gen. 2000, 194–195, 479–485. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, L.; Weng, W.; Wan, H. Preparation of MoVTe(Sb)Nb Mixed Oxide Catalysts Using a Slurry Method for Selective Oxidative Dehydrogenation of Ethane. J. Mol. Catal. A Chem. 2005, 240, 191–196. [Google Scholar] [CrossRef]
- Grasselli, R.K. Selectivity issues in (amm)oxidation catalysis. Catal. Today 2005, 99, 23–31. [Google Scholar] [CrossRef]
- Deniau, B.; Bergeret, G.; Jouguet, B.; Dubois, J.L.; Millet, J.M.M. Preparation of Single M1 Phase MoVTe(Sb)NbO Catalyst: Study of the Effect of M2 Phase Dissolution on the Structure and Catalytic Properties. Top. Catal. 2008, 50, 33–42. [Google Scholar] [CrossRef]
- Nieto, J.L.; Solsona, B.; Concepción, P.; Ivars, F.; Dejoz, A.; Vázquez, M. Reaction products and pathways in the selective oxidation of C2–C4 alkanes on MoVTeNb mixed oxide catalysts. Catal. Today 2010, 157, 291–296. [Google Scholar] [CrossRef]
- Li, X.; Douglas, J.; Douglas, B.; Blom, A.; Vogt, T. Improvement of the Structural Model for the M1 Phase Mo–V–Nb–Te–O Propane (Amm)Oxidation Catalyst. Top. Catal. 2011, 54, 614–626. [Google Scholar] [CrossRef]
- Deniau, B.; Nguyen, T.T.; Delichere, P.; Safonova, O.; Millet, J.M.M. Redox State Dynamics at the Surface of MoVTe(Sb)NbO M1 Phase in Selective Oxidation of Light Alkanes. Top. Catal. 2013, 56, 1952–1962. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Douglas, J.B.; Peter, D., Jr.; James, D.B.; Claus, G.L.; Anthony, F.V.; Weingand, T. Active Centers in Mo–V–Nb–Te–O (amm)Oxidation Catalysts. Catal. Today 2004, 91–92, 251–258. [Google Scholar] [CrossRef]
- Nguyen, T.; Burel, L.; Nguyen, D.; Pham-Huu, C.; Millet, J. Catalytic performance of MoVTeNbO catalyst supported on SiC foam in oxidative dehydrogenation of ethane and ammoxidation of propane. Appl. Catal. A Gen. 2012, 433–434, 41–48. [Google Scholar] [CrossRef]
- Havecker, M.; Wrabetz, S.; Krohnert, J.; Csepei, L.I.; d’Alnoncourt, R.N.; Kolen’ko, Y.V.; Girgsdies, F.; Schlogl, R.; Trunschke, A. Surface Chemistry of Phase-Pure M1 MoVTeNbO Oxide During Operation in Selective Oxidation of Propane to Acrylic Acid. J. Catal. 2012, 285, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, D.; Miracca, I. Dehydrogenation of paraffins: Synergies between catalyst design and reactor engineering. Catal. Today 2006, 111, 133–139. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative Dehydrogenation of Ethane and Propane: How Far from Commercial Implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Schmidt, L.D.; Siddall, J.; Bearden, M. New ways to make old chemicals. AIChE J. 2000, 46, 1492–1495. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.-K.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S.; Cao, L.; Li, W.-X.; Lu, J. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, eaat6413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishanin, I.I.; Bogdan, V.I. Regularities of Oxidative Dehydrogenation of Ethane Over MoVNbTeOx Catalyst Under Supercritical Conditions. Catal. Lett. 2020, 151, 2088–2093. [Google Scholar] [CrossRef]
- Mishanin, I.I.; Viktor, I.B. Advantages of Ethane Oxidative Dehydrogenation on the MoVNbTeOx Catalyst under Elevated Pressure. Mendeleev Commun. 2019, 29, 455–457. [Google Scholar] [CrossRef]
- Chu, B.; Truter, L.; Nijhuis, T.A.; Cheng, Y. Oxidative dehydrogenation of ethane to ethylene over phase-pure M1 MoVNbTeOx catalysts in a micro-channel reactor. Catal. Sci. Technol. 2015, 5, 2807–2813. [Google Scholar] [CrossRef]
- Chen, J.K.; Bollini, P.; Balakotaiah, V. Oxidative Dehydrogenation of Ethane over Mixed Metal Oxide Catalysts: Autothermal or Cooled Tubular Reactor Design? AIChE J. 2021, 67, e17168. [Google Scholar] [CrossRef]
- Galina, A.Z.; Shutilov, A.A.; Bondareva, V.M.; Sobolev, V.I.; Marchuk, A.S.; Tsybulya, S.V.; Prosvirin, I.P.; Ishchenko, A.V.; Gavrilov, V.Y. New Multicomponent MoVSbNbCeOx/SiO2 Catalyst with Enhanced Catalytic Activity for Oxidative Dehydrogenation of Ethane to Ethylene. ChemCatChem 2020, 12, 4149–4159. [Google Scholar]
- Yun, Y.S.; Lee, M.; Sung, J.; Yun, D.; Kim, T.Y.; Park, H.; Lee, K.R.; Song, C.K.; Kim, Y.; Lee, J.; et al. Promoting Effect of Cerium on MoVTeNbO Mixed Oxide Catalyst for Oxidative Dehydrogenation of Ethane to Ethylene. Appl. Catal. B Environ. 2018, 237, 554–562. [Google Scholar] [CrossRef]
- Dang, D.; Chen, X.; Yan, B.H.; Li, Y.K.; Cheng, Y. Catalytic Performance of Phase-Pure M1 MoVTeNbOx/CeO2 Composite for Oxidative Dehydrogenation of Ethane. J. Catal. 2018, 365, 238–248. [Google Scholar] [CrossRef]
- de Arriba, A.; Solsona, B.; Dejoz, A.M.; Concepción, P.; Homs, N.; de la Piscina, P.R.; Nieto, J.M.L. Evolution of the optimal catalytic systems for the oxidative dehydrogenation of ethane: The role of adsorption in the catalytic performance. J. Catal. 2021. [Google Scholar] [CrossRef]
- Annamalai, L.; Ezenwa, S.; Dang, Y.; Tan, H.; Suib, S.L.; Deshlahra, P. Comparison of structural and catalytic properties of monometallic Mo and V oxides and M1 phase mixed oxides for oxidative dehydrogenation. Catal. Today 2020, 368, 28–45. [Google Scholar] [CrossRef]
- Donaubauer, P.J.; Melzer, D.M.; Wanninger, K.; Mestl, G.; Sanchez-Sanchez, M.; Lercher, J.A.; Hinrichsen, O. Intrinsic kinetic model for oxidative dehydrogenation of ethane over MoVTeNb mixed metal oxides: A mechanistic approach. Chem. Eng. J. 2019, 383, 123195. [Google Scholar] [CrossRef]
- Chen, X.; Dang, D.; An, H.; Chu, B.; Cheng, Y. MnO Promoted Phase-Pure M1 MoVTeNb Oxide for Ethane Oxidative Dehydrogenation. J. Taiwan Inst. Chem. Eng. 2019, 95, 103–111. [Google Scholar] [CrossRef]
- Wijaya, K.; Putri, A.R.; Sudiono, S.; Mulijani, S.; Patah, A.; Wibowo, A.C.; Saputri, W.D. Effectively Synthesizing SO4/TiO2 Catalyst and Its Performance for Converting Ethanol into Diethyl Ether (DEE). Catalysts 2021, 11, 1492. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.; Popov, A. Extraction–Pyrolytic Method for TiO2 Polymorphs Production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Xie, P.-J.; Lai, Y.-W.; Lo, A.-Y. Hollow TiO2 Microsphere/Graphene Composite Photocatalyst for CO2 Photoreduction. Catalysts 2021, 11, 1532. [Google Scholar] [CrossRef]
- Holmberg, J.; Haggblad, R.; Andersson, A. A Study of Propane Ammoxidation on Mo–V–Nb–Te-Oxide Catalysts Diluted with Al2O3, SiO2, and TiO2. J. Catal. 2006, 243, 350–359. [Google Scholar] [CrossRef]
- Li, X.; Iglesia, E. Support and promoter effects in the selective oxidation of ethane to acetic acid catalyzed by Mo-V-Nb oxides. Appl. Catal. A Gen. 2008, 334, 339–347. [Google Scholar] [CrossRef]
- Che-Galicia, G.; Ruiz-Martínez, R.S.; López-Isunza, F.; Castillo-Araiza, C.O. Modeling of Oxidative Dehydrogenation of Ethane to Ethylene on a MoVNbTeO/TiO2 Catalyst in an Industrial-Scale Packed Bed Catalytic Reactor. Chem. Eng. J. 2015, 280, 682–694. [Google Scholar] [CrossRef]
- Dang, D.; Chen, Y.; Chen, X.; Feng, K.; Yan, B.; Cheng, Y. Phase-pure M1 MoVNbTeOx/TiO2 nanocomposite catalysts: Highly catalytic performance for oxidative dehydrogenation of ethane. Catal. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Chu, B.; Truter, L.; Nijhuis, T.A.; Cheng, Y. Performance of Phase-Pure M1 MoVNbTeOx Catalysts by Hydrothermal Synthesis with Different Post-Treatments for the Oxidative Dehydrogenation of Ethane. Appl. Catal. A Gen. 2015, 498, 99–106. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Chu, B.; An, H.; Cheng, Y. Valence Variation of Phase-Pure M1 MoVNbTeOx Oxide by Plasma Treatment for Improved Catalytic Performance in Oxidative Dehydrogenation of Ethane. RSC Adv. 2015, 5, 91295–91301. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Berger, R.J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. The Six-Flow Reactor Technology—A Review on Fast Catalyst Screening and Kinetic Studies. Catal. Today 2000, 60, 93–109. [Google Scholar] [CrossRef]
- Solsona, B.; Vazquez, M.; Ivars, F.; Dejoz, A.; Concepcion, P.; Lopeznieto, J. Selective Oxidation of Propane and Ethane on Diluted Mo–V–Nb–Te Mixed-Oxide Catalysts. J. Catal. 2007, 252, 271–280. [Google Scholar] [CrossRef]
- Huerta, M.V.M.; Gao, X.; Tian, H.; Wachs, I.; Fierro, J.; Bañares, M. Oxidative dehydrogenation of ethane to ethylene over alumina-supported vanadium oxide catalysts: Relationship between molecular structures and chemical reactivity. Catal. Today 2006, 118, 279–287. [Google Scholar]
- Grant, J.T.; Venegas, J.M.; McDermott, W.; Hermans, I. Aerobic Oxidations of Light Alkanes over Solid Metal Oxide Catalysts. Chem. Rev. 2017, 118, 2769–2815. [Google Scholar] [CrossRef]
- Andrushkevich, T.V.; Popova, G.Y.; Chesalov, Y.A.; Ischenko, E.V.; Khramov, M.I.; Kaichev, V.V. Propane Ammoxidation on Bi Promoted MoVTeNbOx Oxide Catalysts: Effect of Reaction Mixture Composition. Appl. Catal. A Gen. 2015, 506, 109–117. [Google Scholar] [CrossRef]
- Botella, P.; García-González, E.; Nieto, J.M.L.; Gonzalez-Calbet, J.M. MoVTeNbO multifunctional catalysts: Correlation between constituent crystalline phases and catalytic performance. Solid State Sci. 2005, 7, 507–519. [Google Scholar] [CrossRef]
- Tao, F.; Shen, Y.; Liang, Y.; Li, H. Synthesis and Characterization of Co(OH)2/TiO2 Nanotube Composites as Supercapacitor Materials. J. Solid State Electrochem. 2006, 11, 853–858. [Google Scholar] [CrossRef]
- Cheng, M.J.; Goddard, W.A., 3rd. In Silico Design of Highly Selective Mo-V-Te-Nb-O Mixed Metal Oxide Catalysts for Ammoxidation and Oxidative Dehydrogenation of Propane and Ethane. J. Am. Chem. Soc. 2015, 137, 13224–13227. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.S.; Armendáriz-Herrera, H.; Solorzano, R.Q.; Del Ángel, P.; Nava, N.; Massó, A.; Nieto, J.M.L. Chemical, Structural, and Morphological Changes of a MoVTeNb Catalyst during Oxidative Dehydrogenation of Ethane. ACS Catal. 2014, 4, 1292–1301. [Google Scholar] [CrossRef]
- Aouine, M.; Epicier, T.; Millet, J.-M.M. In Situ Environmental STEM Study of the MoVTe Oxide M1 Phase Catalysts for Ethane Oxidative Dehydrogenation. ACS Catal. 2016, 6, 4775–4781. [Google Scholar] [CrossRef]
- Guan, J.; Wu, S.; Wang, H.; Jing, S.; Wang, G.; Zhen, K.; Kan, Q. Synthesis and characterization of MoVTeCeO catalysts and their catalytic performance for selective oxidation of isobutane and isobutylene. J. Catal. 2007, 251, 354–362. [Google Scholar] [CrossRef]
- Demeter, M.; Neumann, M.; Reichelt, W. Mixed-valence vanadium oxides studied by XPS. Surf. Sci. 2000, 454-456, 41–44. [Google Scholar] [CrossRef]
- Botella, P.; Dejoz, A.; Abello, M.C.; Vázquez, M.I.; Arrúa, L.; Nieto, J.M.L. Selective Oxidation of Ethane: Developing an Orthorhombic Phase in Mo–V–X (X=Nb, Sb, Te) Mixed Oxides. Catal. Today 2009, 142, 272–277. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, C.; Xu, C.; Li, H.; Huang, H.; Song, L.; Li, X. Kinetics study for the oxidative dehydrogenation of ethyl lactate to ethyl pyruvate over MoVNbO based catalysts. Chem. Eng. J. 2016, 296, 217–224. [Google Scholar] [CrossRef]
- Liu, J.; Mohamed, F.; Sauer, J. Selective oxidation of propene by vanadium oxide monomers supported on silica. J. Catal. 2014, 317, 75–82. [Google Scholar] [CrossRef]
- Kwon, S.; Deshlahra, P.; Iglesia, E. Dioxygen activation routes in Mars-van Krevelen redox cycles catalyzed by metal oxides. J. Catal. 2018, 364, 228–247. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, J.; Huo, Y.N.; Bian, Z.F.; Li, H.X. Nanocrystalline Fe/TiO2 Visible Photocatalyst with a Mesoporous Structure Prepared Via a Nonhydrolytic Sol-Gel Route. J. Phys. Chem. C 2007, 111, 18965–18969. [Google Scholar] [CrossRef]
- Feng, K.; Wang, S.; Zhang, D.; Wang, L.; Yu, Y.; Feng, K.; Li, Z.; Zhu, Z.; Li, C.; Cai, M.; et al. Cobalt Plasmonic Superstructures Enable Almost 100% Broadband Photon Efficient CO2 Photocatalysis. Adv. Mater. 2020, 32, 2000014. [Google Scholar] [CrossRef]














| Catalyst | α | β | ODHE Ea (kJ/mol) |
|---|---|---|---|
| M1 | 0.90 | 0.02 | 91.4 |
| M1-40TiO2-SM | 0.99 | 0.06 | 82.3 |
| M1-40TiO2-PM | 0.90 | 0.06 | 88.4 |
| Catalyst | TiO2 Content (wt %) | Bulk Composition |
|---|---|---|
| M1 | - | MoV0.25Nb0.24Te0.12 |
| M1-40TiO2-SM | 39.6 | MoV0.25Nb0.24Te0.12 |
| M1-40TiO2-PM | 40.0 | MoV0.25Nb0.24Te0.12 |
| Catalysts | Specific Surface Area (m2/g) | Pore Volume (cc/g) |
|---|---|---|
| M1 | 17.7 | 0.060 |
| Used M1 | 15.7 | 0.048 |
| M1-40TiO2-SM | 52.4 | 0.085 |
| Used M1-40TiO2-SM | 49.6 | 0.080 |
| M1-40TiO2-PM | 36.3 | 0.066 |
| Used M1-40TiO2-PM | 36.1 | 0.063 |
| TiO2 | 52.7 | 0.104 |
| Catalyst | V5+/V4+ Ratio | Surface Composition |
|---|---|---|
| M1 | 1.00 | MoV0.10Nb0.34Te0.21 |
| Used M1 | 1.00 | MoV0.10Nb0.34Te0.21 |
| M1-40TiO2-SM | 1.18 | MoV0.11Nb0.33Te0.34 |
| Used M1-40TiO2-SM | 0.87 | MoV0.11Nb0.32Te0.32 |
| M1-40TiO2-PM | 1.10 | MoV0.11Nb0.36Te0.21 |
| Used M1-40TiO2-PM | 1.10 | MoV0.11Nb0.36Te0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Dang, D.; Yan, B.; Cheng, Y. Mixed Metal Oxides of M1 MoVNbTeOx and TiO2 as Composite Catalyst for Oxidative Dehydrogenation of Ethane. Catalysts 2022, 12, 71. https://doi.org/10.3390/catal12010071
Chen Y, Dang D, Yan B, Cheng Y. Mixed Metal Oxides of M1 MoVNbTeOx and TiO2 as Composite Catalyst for Oxidative Dehydrogenation of Ethane. Catalysts. 2022; 12(1):71. https://doi.org/10.3390/catal12010071
Chicago/Turabian StyleChen, Yuxin, Dan Dang, Binhang Yan, and Yi Cheng. 2022. "Mixed Metal Oxides of M1 MoVNbTeOx and TiO2 as Composite Catalyst for Oxidative Dehydrogenation of Ethane" Catalysts 12, no. 1: 71. https://doi.org/10.3390/catal12010071
APA StyleChen, Y., Dang, D., Yan, B., & Cheng, Y. (2022). Mixed Metal Oxides of M1 MoVNbTeOx and TiO2 as Composite Catalyst for Oxidative Dehydrogenation of Ethane. Catalysts, 12(1), 71. https://doi.org/10.3390/catal12010071