Eco-Friendly Synthesis of Organo-Functionalized Mesoporous Silica for the Condensation Reaction
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalyst Characterisation
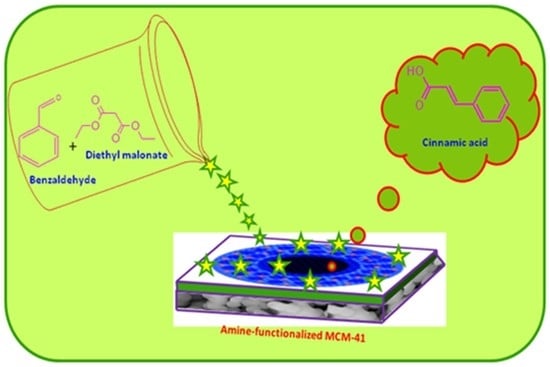
3.3. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erigoni, A.; Diaz, U. Porous Silica-Based Organic-Inorganic Hybrid Catalysts: A Review. Catalysts 2021, 11, 79. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Gadea-Ramos, E.; Kaneko, K.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. High-Pressure Methane Storage in Porous Materials: Are Carbon Materials in the Pole Position? Chem. Mater. 2015, 27, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Brotchie, A. Porous materials: MOFs go fungal. Nat. Rev. Mater. 2016, 1, 15015. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. Energy Chem. 2019, 1, 100006. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Salama, R.S.; El-Bahy, S.M.; Mannaa, M.A. Sulfamic acid supported on mesoporous MCM-41 as a novel, efficient and reusable heterogeneous solid acid catalyst for synthesis of xanthene, dihydropyrimidinone and coumarin derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127261. [Google Scholar] [CrossRef]
- El-Hakam, S.A.; ALShorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeeda, W.S.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of 12-tungestophosphoric acid supported on Zr/MCM-41 composite with excellent heterogeneous catalyst and promising adsorbent of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127753. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic-Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Rana, S.; Parida, K.M. Organic amine-functionalized silica-based mesoporous materials: An update of syntheses and catalytic applications. RSC Adv. 2014, 4, 57111. [Google Scholar] [CrossRef]
- Parida, K.; Rana, S.; Mallick, S.; Rath, D. Cesium salts of heteropoly acid immobilised mesoporous silica: An efficient catalyst for acylation of anisole. J. Colloid Interface Sci. 2010, 350, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Mallick, S.; Mohapatra, L.; Varadwaj, G.B.B.; Parida, K. A facile method for synthesis of Keggin-type cesium salt of iron substituted lacunary phosphotungstate supported on MCM-41 and study of its extraordinary catalytic activity. Catalysis Today 2012, 198, 52–58. [Google Scholar] [CrossRef]
- Nale, D.B.; Rana, S.; Parida, K.; Bhanage, B.M. Amine functionalized MCM-41: An efficient heterogeneous recyclable catalyst for the synthesis of quinazoline-2, 4 (1 H, 3 H)-diones from carbon dioxide and 2-aminobenzonitriles in water. Catal. Sci. Technol. 2014, 4, 1608–1614. [Google Scholar] [CrossRef]
- Lim, M.H.; Stein, A. Comparative Studies of Grafting and Direct Syntheses of Inorganic–Organic Hybrid Mesoporous Materials. Chem. Mater. 1999, 11, 3285–3295. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Karakostas, T.; Petridis, D. “Side Chain” Modification of MCM-41 Silica through the Exchange of the Surfactant Template with Charged Functionalized Organosiloxanes: An Efficient Route to Valuable Reconstructed MCM-41 Derivatives. J. Phys. Chem. B 2003, 107, 920–925. [Google Scholar] [CrossRef]
- Brunel, D.; Blanc, A.C.; Garrone, E.; Onida, B.; Rocchia, M.; Nagy, J.B.; Macquarrie, D.J. Spectroscopic studies on aminopropyl-containing micelle templated silicas. Comparison of grafted and co-condensation routes. Stud. Surf. Sci. Catal. 2002, 142, 1395–1402. [Google Scholar]
- Liao, Q.; Zeng, L.; Li, L.; Guo, F.; Wu, L.; Le, S. Synthesis of functionalised mesoporous silica and its application for Cu(II) removal. Desalin. Water Treat. 2014, 56, 2154–2159. [Google Scholar] [CrossRef]
- Atoub, N.; Amiri, A.; Badiei, A.; Ghasemi, J.B. One-Pot Hydrothermal Synthesis of Functionalized Mesoporous Silica for Effective Removal of Pb(II) Ion. J. Nanostruct. 2021, 11, 125–135. [Google Scholar]
- Yang, Y.; Yao, H.F.; Xi, F.G.; Gao, E.Q. Amino-functionalized Zr(IV) metal-organic framework as bifunctional acid-base catalyst for Knoevenagel condensation. J. Mol. Catal. A Chem. 2014, 390, 198–205. [Google Scholar] [CrossRef]
- Ren, Y.; Lu, J.; Jiang, O.; Cheng, X.; Chen, J. Amine-grafted on lanthanide metal-organic frameworks: Three solid base catalysts for Knoevenagel condensation reaction. Chin. J. Catal. 2015, 36, 1949–1956. [Google Scholar] [CrossRef]
- Varadwaj, G.B.; Rana, S.; Parida, K.M. Amine functionalised K10 montmorillonite: A solid acid-base catalyst for the Knoevenagel condensation reaction. Dalton Trans. 2013, 42, 5122–5129. [Google Scholar] [CrossRef]
- Rana, S.; Jonnalagadda, S.B. Synthesis and characterisation of amine-functionalised graphene oxide and scope as catalyst for Knoevenagel condensation reaction. Catal. Commun. 2017, 92, 31–34. [Google Scholar] [CrossRef]
- Wang, X.G.; Lin, K.S.K.; Chan, J.C.C.; Cheng, S.F. Direct Synthesis and Catalytic Applications of Ordered Large Pore Aminopropyl-Functionalized SBA-15 Mesoporous Materials. J. Phys. Chem. B 2005, 109, 1763–1769. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Nakamura, T.; Fukumoto, K.; Yamamoto, M.; Akimoto, Y.; Akimoto, Y.; Yano, K. Direct synthesis of amino functionalised monodispersed mesoporous silica spheres and their catalytic activity for nitroaldol condensation. J. Mol. Catal. A Chem. 2008, 280, 224–232. [Google Scholar] [CrossRef]
- Neogi, S.; Sharma, M.K.; Bharadwaj, P.K. Knoevenagel condensation and cyanosilylation reactions catalysed by a MOF containing coordinatively unsaturated Zn(II) centers. J. Mol. Catal. A Chem. 2009, 299, 1–4. [Google Scholar] [CrossRef]
- Parida, K.M.; Rath, D. Amine functionalised MCM-41: An active and reusable catalyst for Knoevenagel condensation reaction. J. Mol. Catal. A Chem. 2009, 310, 93–100. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Sreekanth, P.; Bandopadhyay, T.; Figueras, F.; Tuel, A. Knoevenagel and aldol condensations catalysed by a new diamino-functionalised mesoporous material. J. Mol. Catal. A Chem. 1999, 142, 361–365. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.; Tong, M.; Xiao, Y.; Li, H.; Zhang, F. Primary amine-functionalised mesoporous phenolic resin as an effective and stable solid base catalyst for Knoevenagel reactions in water. Green Synth. Catal. 2020, 1, 79–82. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Saliy, O.; Hu, W.; Wang, J. Robust Amino-Functionalized Mesoporous Silica Hollow Spheres Templated by CO2 Bubbles. Molecules 2022, 27, 53. [Google Scholar] [CrossRef]
- Zhong, L.W.; Su, B.; Guo, Y.J.; Chu, L.P. Preparation and synergetic catalytic effects of amino-functionalised MCM-41 catalysts. Sci. China Chem. 2012, 55, 1167–1174. [Google Scholar]
- Mikhail, D.; Stepanenko, Y.; Dzenis, Y.; Karotki, A.; Rebane, A.; Taylor, P.N.; Anderson, H.L. Extremely Strong Near-IR Two-Photon Absorption in Conjugated Porphyrin Dimers: Quantitative Description with Three-Essential-States Model. J. Phys. Chem. B 2005, 109, 7223–7236. [Google Scholar]
- Ebrahimi-Gatkash, M.; Younesi, H.; Shahbazi, A.; Heidari, A. Amino-functionalized mesoporous MCM-41 silica as an efficient adsorbent for water treatment: Batch and fixed-bed column adsorption of the nitrate anion. Appl. Water Sci. 2017, 7, 1887–1901. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, Z.; Zinatizadeh, A.A.; Zinadini, S.; van Loosdrecht, M.; Younesi, H. A new anti-fouling polysulphone nanofiltration membrane blended by amine-functionalised MCM-41 for post treating waste stabilisation pond’s effluent. J. Environ. Manag. 2021, 290, 112649. [Google Scholar] [CrossRef] [PubMed]
- Matei, C.; Buhǎlţeanu, L.; Berger, D.; Mitran, R. Functionalized mesoporous silica as matrix for shape-stabilised phase change materials. Int. J. Heat Mass Transf. 2019, 144, 118699. [Google Scholar] [CrossRef]
- Rana, S.; Mallick, S.; Parida, K.M. Selective oxidation of benzaldehyde by molecular oxygen over molybdovanadophosphoric acid supported MCM-41. J. Porous Mater. 2012, 19, 397–404. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Rath, D.; Rana, S.; Parida, K.M. CuxH3-2xPW12O40 Supported on MCM-41: Their Activity to Heck Vinylation of Aryl Halides. Ind. Eng. Chem. Res. 2010, 49, 8942–8948. [Google Scholar] [CrossRef]
- Kubota, Y.; Nishizaki, Y.; Ikeya, H.; Saeki, M.; Hida, T.; Kawazu, S.; Yoshida, M.; Fujii, H.; Sugi, Y. Organic–silicate hybrid catalysts based on various defined structures for Knoevenagel condensation. Microporous Mesoporous Mater. 2004, 70, 135–149. [Google Scholar] [CrossRef]
- Macquarrie, D.J.; Jackson, D.B. Aminopropylated MCMs as base catalysts: A comparison with aminopropylated silica. Chem. Commun. 1997, 1781–1782. [Google Scholar] [CrossRef]






| Sl. No. | Catalyst | Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|
| Cinnamic Acid | Other | |||
| 1 | Without catalyst | 0 | 0 | 0 |
| 2 | MCM-41 | 10 | 51 | 49 |
| 3 | 12.8 A-MCM-41-S | 95 | 98 | 2 |
| 4 | 12.8 A-MCM-41-C | 92.4 | 98 | 2 |
| Sl. No. |  Reactant |  Major Product | Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|---|
| Major | Others | ||||
| 1 | X = CHO | X = CH = CH-COOH | 95 | 98 | 2 |
| 2 | X = CHO, W = NH2 | X = CH = CH-COOH, W = NH2 | 84 | 92 | 8 |
| 3 | X = CHO, Z = OH | X = CH = CH-COOH, Z = OH | 89 | 91 | 9 |
| 4 | X = CHO, W = CH3 | X = CH = CH-COOH, W = CH3 | 77 | 85 | 15 |
| 5 | X = CHO, Y = OH | X = CH = CH-COOH, Y = OH | 85 | 89 | 11 |
| 6 | X = CHO, W = F | X = CH = CH-COOH, W = F | 92 | 93 | 7 |
| 7 | X = CHO, W = NO2 | X = CH = CH-COOH, W = NO2 | 99 | 99 | 1 |
| 8 | X = CHO, W = COH3 | X = CH = CH-COOH, W = COH3 | 79 | 83 | 17 |
| 9 | X = CH = CH-CHO | X = CH = CH-CH = CH-COOH | 97 | 98 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, S.; Velázquez, J.J.; Jonnalagadda, S.B. Eco-Friendly Synthesis of Organo-Functionalized Mesoporous Silica for the Condensation Reaction. Catalysts 2022, 12, 1212. https://doi.org/10.3390/catal12101212
Rana S, Velázquez JJ, Jonnalagadda SB. Eco-Friendly Synthesis of Organo-Functionalized Mesoporous Silica for the Condensation Reaction. Catalysts. 2022; 12(10):1212. https://doi.org/10.3390/catal12101212
Chicago/Turabian StyleRana, Surjyakanta, José J. Velázquez, and Sreekantha B. Jonnalagadda. 2022. "Eco-Friendly Synthesis of Organo-Functionalized Mesoporous Silica for the Condensation Reaction" Catalysts 12, no. 10: 1212. https://doi.org/10.3390/catal12101212
APA StyleRana, S., Velázquez, J. J., & Jonnalagadda, S. B. (2022). Eco-Friendly Synthesis of Organo-Functionalized Mesoporous Silica for the Condensation Reaction. Catalysts, 12(10), 1212. https://doi.org/10.3390/catal12101212