Visible Light Active Magnesium Silicate–Graphitic Carbon Nitride Nanocomposites for Methylene Blue Degradation and Pb2+ Adsorption
Abstract
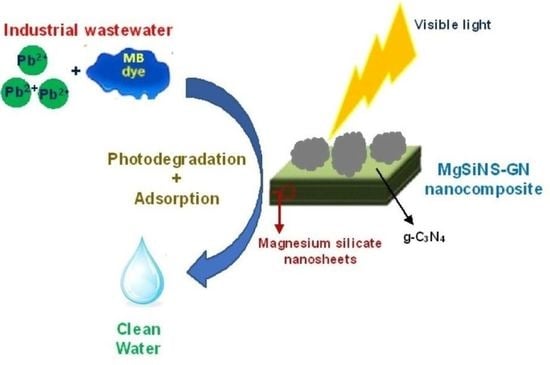
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Synthesis of Nanomaterials
3.2.1. Magnesium Silicate Nanosheets (MgSiNS)
3.2.2. Graphitic Carbon Nitride (g-C3N4)
3.2.3. MgSiNS/g-C3N4 Nanocomposites
3.3. Characterization of Samples
3.4. Photocatalytic Degradation of Methylene Blue
3.5. Pb2+ Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar Reddy, D.; Lee, S. Water pollution and treatment technologies. J. Environ. Anal. Toxicol. 2012, 2, e103. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Angove, M.J.; Aryal, R.; Abuel-Naga, H.; Mainali, B. Removal of natural organic matter from source water: Review on coagulants, dual coagulation, alternative coagulants, and mechanisms. J. Water Process Eng. 2021, 40, 101820. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Qu, J.; Li, W.; Cao, C.; Yin, X.; Zhao, L.; Bai, J.; Qin, Z.; Song, W. Metal silicate nanotubes with nanostructured walls as superb adsorbents for uranyl ions and lead ions in water. J. Mater. Chem. 2012, 22, 17222–17226. [Google Scholar] [CrossRef]
- Souza, M.T.; Peñarrieta-Juanito, G.M.; Henriques, B.; Silva, F.S.; Oliveira, A.P.; Souza, J.C. Lithium-zirconium silicate glass-ceramics for restorative dentistry: Physicochemical analysis and biological response in contact with human osteoblast. Materialia 2018, 2, 37–45. [Google Scholar] [CrossRef]
- Narasimharao, K.; Mokhtar, M.; Basahel, S.N.; Al-Thabaiti, S.A. Synthesis, characterization, and catalytic activity of nitridated magnesium silicate catalysts. J. Mater. Sci. 2013, 48, 4274–4283. [Google Scholar] [CrossRef]
- Yan, T.; Li, N.; Qiao, Z.; Li, W.; Wang, H.; Jing, Z.; Yu, Y.; Jiang, Z. Ultrathin sodium ferric silicate 2D nanosheets: A novel and robust adsorbent for selective removal of cationic dyes in wastewater. J. Alloys Compd. 2019, 784, 256–265. [Google Scholar] [CrossRef]
- Wang, L.; Bahnemann, D.W.; Bian, L.; Dong, G.; Zhao, J.; Wang, C. Two-dimensional layered zinc silicate nanosheets with excellent photocatalytic performance for organic pollutant degradation and CO2 conversion. Angew. Chem. 2019, 131, 8187–8192. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.J.; Chen, F. Ultrathin Calcium Silicate Hydrate Nanosheets with Large Specific Surface Areas: Synthesis, Crystallization, Layered Self-Assembly and Applications as Excellent Adsorbents for Drug, Protein, and Metal Ions. Small 2013, 9, 2911–2925. [Google Scholar] [CrossRef]
- Rashid, I.; Daraghmeh, N.H.; Al-omari, M.M.; Chowdhery, B.Z.; Leharne, S.A.; Hodali, H.A.; Badwan, A.A. Magnesium silicate. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 241–285. [Google Scholar] [CrossRef]
- Ezeibe, M.; Egbuji, A.; Okoroafor, O.; Eze, J.; Ijabo, O.; Ngene, A.; Eze, I.; Ugonabo, J.; Sanda, M.; Mbuko, I. Antiviral Effects of a Synthetic Aluminium-Magnesium Silicate on Avian Influenza Virus. Nat. Prec. 2011. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, J.; Qi, Y.; Wang, F.; Hong, W.; Li, H.; Jiang, Y. Facile preparation of hydroxyl-rich mesoporous magnesium silicate with excellent adsorption performance. Surf. Interfaces 2020, 20, 100519. [Google Scholar] [CrossRef]
- Huang, R.; He, L.; Zhang, T.; Li, D.; Tang, P.; Zhao, Y.; Feng, Y. Fabrication and adsorption behavior of magnesium silicate hydrate nanoparticles towards methylene blue. Nanomaterials 2018, 8, 271. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Yang, Y.; Xiang, G.; Wang, X. Magnesium silicate hollow nanostructures as highly efficient absorbents for toxic metal ions. J. Phys. Chem. C 2009, 113, 10441–10445. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.; Zhou, H.; Zhang, Y.; Wang, X. Two-dimensional lamellar magnesium silicate with large spacing as an excellent adsorbent for uranium immobilization. Environ. Sci. Nano 2018, 5, 2406–2414. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabinerio, S.A. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride-based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhu, Y. Nanoporous graphitic carbon nitride with enhanced photocatalytic performance. Langmuir 2013, 29, 10566–10572. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhamg, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Chao, S.; Sun, B.; Zang, N.; Qiu, J.; Wang, C.; Li, X. A flexible magnesium silicate coated electrospun fiber adsorbent for high-efficiency removal of a toxic cationic herbicide. New J. Chem. 2017, 41, 15601–15611. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, J.; Jiang, B. Rational design of hierarchical macroporous–mesoporous magnesium silicate for highly efficient removal of organic dye and Pb 2+. RSC Adv. 2017, 7, 47225–47234. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, T.; Nayeri, S.; Hamidi, N. Graphitic carbon nitride (g-C3N4)/graphite nanocomposite as an extraordinarily sensitive sensor for sub-micromolar detection of oxalic acid in biological samples. RSC Adv. 2019, 9, 13096–13103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherrer, P.; Nachricht, G. Bestimmung der grosse und der innerenstruktur von kolloidteilchen mittels rontgenstrahlen, nachrichten von der gesellschaft der wissenschaften, gottingen. Math.-Phys. Kl. 1918, 2, 98–100. Available online: http://resolver.sub.uni-goettingen.de/purl?PPN252457811_1918 (accessed on 13 October 2022).
- Ossman, M.; Mansour, M.; Fattah, M.; Taha, N.; Kiros, Y. Peanut shells and talc powder for removal of hexavalent chromium from aqueous solutions. Bulg. Chem. Commun. 2014, 3, 629–639. [Google Scholar]
- Tonelli, M.; Martini, F.; Calucci, L.; Fratini, E.; Geppi, M.; Ridi, F.; Borsacchi, S.; Baglioni, P. Structural characterization of magnesium silicate hydrate: Towards the design of eco-sustainable cements. Dalton Trans. 2016, 45, 3294–3304. [Google Scholar] [CrossRef]
- Sun, T.-W.; Zhu, Y.-J.; Qi, C.; Chen, F.; Jiang, Y.-Y.; Zhang, Y.-G.; Wu, J.; Wi, C. Templated solvothermal synthesis of magnesium silicate hollow nanospheres with ultrahigh specific surface area and their application in high-performance protein adsorption and drug delivery. J. Mater. Chem. B 2016, 4, 3257–3268. [Google Scholar] [CrossRef]
- Kuterasiński, Ł.; Wojtkiewicz, A.M.; Sadowska, M.; Żeliszewska, P.; Napruszewska, B.D.; Zimowska, M.; Pytlik, M.; Biessikirski, A. Variously Prepared Zeolite Y as a Modifier of ANFO. Materials 2022, 15, 5855. [Google Scholar] [CrossRef]
- Martha, S.; Nashim, A.; Parida, K. Facile synthesis of highly active gC 3 N 4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816–7824. [Google Scholar] [CrossRef]
- Yang, J.; Wu, X.; Li, X.; Liu, Y.; Gao, M.; Liu, X.; Kong, L.; Yang, S. Synthesis and characterization of nitrogen-rich carbon nitride nanobelts by pyrolysis of melamine. Appl. Phys. A 2011, 105, 161–166. [Google Scholar] [CrossRef]
- Li, W.; Chen, Q.; Zhong, Q. One-pot fabrication of mesoporous g-C3N4/NiS co-catalyst counter electrodes for quantum-dot-sensitized solar cells. J. Mater. Sci. 2020, 55, 10712–10724. [Google Scholar] [CrossRef]
- Dolgonos, A.; Mason, T.O.; Poeppelmeier, K.R. Direct optical band gap measurement in polycrystalline semiconductors: A critical look at the Tauc method. J. Solid State Chem. 2016, 240, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Medrano, V.G.B.; Celis, V.N.; Giraldo, R.I. Systematic Analysis of the Nitrogen Adsorption-Desorption Isotherms Recorded for a Series of Microporous—Mesoporous Amorphous Aluminosilicates Using Classical Methods; ChemRxiv–Cambridge Open Engage: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, D.; Duan, X.; Hong, W.; Liang, J. Functionalized nanoflower-like hydroxyl magnesium silicate for effective adsorption of aflatoxin B1. J. Hazard. Mater. 2020, 387, 121792. [Google Scholar] [CrossRef] [PubMed]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Waltham, MA, USA, 1992; p. 261. [Google Scholar]
- Zhang, Y.; Zhu, L.; Chen, L.; Liu, L.; Ye, G. Influence of Magnesia on Demoulding Strength of Colloidal Silica-Bonded Castables. Rev. Adv. Mater. Sci. 2019, 58, 32–37. [Google Scholar] [CrossRef]
- Ma, J.W.; Lee, W.J.; Bae, J.M.; Jeong, K.S.; Oh, S.H.; Kim, J.H.; Kim, S.-H.; Seo, J.-H.; Ahn, J.-P.; Kim, H.; et al. Carrier mobility enhancement of tensile strained Si and SiGe nanowires via surface defect engineering. Nano Lett. 2015, 15, 7204–7210. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, Q.; Javed, M.S.; Gong, Y.; Aslam, M.K.; Chen, C. The effects of NaF concentration on electrochemical and corrosion behavior of AZ31B magnesium alloy in a composite electrolyte. RSC Adv. 2017, 7, 5880–5887. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Fischer, A.; Goettmenn, F.; Antonietti, M.; Mȕller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Meng, J.; Pei, J.; He, Z.; Wu, S.; Lin, Q.; Wei, X.; Li, J.; Zhang, Z. Facile synthesis of g-C3N4 nanosheets loaded with WO3 nanoparticles with enhanced photocatalytic performance under visible light irradiation. RSC Adv. 2017, 7, 24097–24104. [Google Scholar] [CrossRef]
- Solehudin, M.; Sirimahachai, U.; Ali, G.A.M.; Chong, K.F.; Wongnawa, S. One-pot synthesis of isotype heterojunction g-C3N4-MU photocatalyst for effective tetracycline hydrochloride antibiotic and reactive orange 16 dye removal. Adv. Powder Technol. 2020, 31, 1891–1902. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Jasińska, A.; Soboń, A.; Bińkowska, A.G.; Dlugoński, J. Analysis of decolorization potential of Myrothecium roridum in the light of its secretome and toxicological studies. Environ. Sci. Pollut. Res. 2019, 26, 26313–26323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.-Z.; Qi, N.; Chen, W.-J.; Zhao, H. Construction of hydroxyethyl cellulose/silica/graphitic carbon nitride solid foam for adsorption and photocatalytic degradation of dyes. Arab. J. Chem. 2022, 15, 104105. [Google Scholar] [CrossRef]
- Li, H.; Cai, L.; Wang, X.; Shi, H. Fabrication of AgCl/Ag3PO4/graphitic carbon nitride heterojunctions for enhanced visible light photocatalytic decomposition of methylene blue, methylparaben and E. coli. RSC Adv. 2021, 11, 6383–6394. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, J.; Sun, C.; Ling, Y.; Li, S.; Li, X.; Zhao, J.; Yang, K. La2O3-modified graphite carbon nitride achieving the enhanced photocatalytic degradation of different organic pollutants under visible light irradiation. Mater.Chem. Phys. 2020, 246, 122846. [Google Scholar] [CrossRef]
- Subashini, A.; Varun Prasath, P.; Sagadevan, S.; Anita Lett, J.; Fatimah, I.; Mohammad, F.; Al-Lohedan, H.A.; Alshahateet, S.F.; Oh, W.C. Enhanced photocatalytic degradation efficiency of graphitic carbon nitride-loaded CeO2 nanoparticles. Chem. Phy. Lett. 2021, 769, 138441. [Google Scholar] [CrossRef]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.I.; Raynaud, P.; Nada, A.A. Elaboration of Fe3O4/ZnO nanocomposite with highly performance photocatalytic activity for degradation methylene blue under visible light irradiation. Environ. Technol. Innov. 2021, 23, 101710. [Google Scholar] [CrossRef]
- Hung, N.V.; Nguyet, B.T.M.; Nghi, N.H.; Nguyen, V.T.; Binh, T.V.; Tu, N.T.T.; Dung, N.N.; Khieu, D.Q. Visible light photocatalytic degradation of organic dyes using W-modified TiO2/SiO2 catalyst. Vietnam J. Chem. 2021, 59, 620–638. [Google Scholar] [CrossRef]
- Hakimi-Tehrani, M.J.; Hassanzadeh-Tabrizi, S.A.; Koupaei, N.; Saffar-Teluri, A.; Rafiei, M. Facile thermal synthesis of g–C3N4/ZnO nanocomposite with antibacterial properties for photodegradation of Methylene blue. Mater. Res. Exp. 2021, 8, 125002. [Google Scholar] [CrossRef]
- Chen, Q.; Dou, H.; Zheng, S.; Rao, X.; Zhang, Y. Photocatalytic H2 evolution and MB degradation over nickel-doped graphitic carbon nitride microwires under visible light irradiation. J. Photochem. Photobio. A Chem. 2019, 382, 111931. [Google Scholar] [CrossRef]
- Zhang, X.; Shakeel, M.; Li, B.; Zhang, J.; Wang, L. Synthesis of foamed zinc oxide–silica spheres coupled with g-C3N4 nanosheets for visible light photocatalysis. J. Mater. Sci. 2019, 54, 13118–13134. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Highly reusable visible light active hierarchical porous WO3/SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]












| Sample | BET Surface Area (m2/g) | Average Pore Size (nm) | Pore Volume (cc/g) | Crystal Size (nm) | |
|---|---|---|---|---|---|
| MgSiNS | g-C3N4 | ||||
| MgSiNS | 294 | 3.4 | 0.103 | 2.50 | - |
| g−C3N4 | 121 | 3.8 | 0.132 | 4.16 | 5.53 |
| MgSiNS−GN5 | 186 | 4.8 | 0.077 | 4.19 | - |
| MgSiNS−GN10 | 159 | 4.3 | 0.060 | 4.41 | - |
| MgSiNS−GN15 | 116 | 3.9 | 0.040 | 5.34 | 5.12 |
| MgSiNS−GN20 | 108 | 3.8 | 0.037 | 5.41 | 5.03 |
| MgSiNS−GN25 | 88 | 3.9 | 0.009 | - | 4.89 |
| Composite | MgSiNS (wt %) | g-C3N4 (wt %) | * Elemental Composition (Mass %) | ||||
|---|---|---|---|---|---|---|---|
| Mg | Si | O | C | N | |||
| MgSiNS-GN5 | 5 | 95 | 20.8 | 22.2 | 44.4 | 5.9 | 6.7 |
| MgSiNS-GN10 | 10 | 90 | 18.3 | 19.5 | 40.5 | 10.5 | 11.2 |
| MgSiNS-GN15 | 15 | 85 | 16.5 | 17.7 | 35.3 | 14.8 | 15.7 |
| MgSiNS-GN20 | 20 | 80 | 14.6 | 16.1 | 32.1 | 18.1 | 19.1 |
| MgSiNS-GN25 | 25 | 75 | 12.7 | 13.2 | 30.4 | 20.4 | 22.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnassar, M.A.; Alshehri, A.; Narasimharao, K. Visible Light Active Magnesium Silicate–Graphitic Carbon Nitride Nanocomposites for Methylene Blue Degradation and Pb2+ Adsorption. Catalysts 2022, 12, 1256. https://doi.org/10.3390/catal12101256
Alnassar MA, Alshehri A, Narasimharao K. Visible Light Active Magnesium Silicate–Graphitic Carbon Nitride Nanocomposites for Methylene Blue Degradation and Pb2+ Adsorption. Catalysts. 2022; 12(10):1256. https://doi.org/10.3390/catal12101256
Chicago/Turabian StyleAlnassar, Muhmmed Ali, Abdulmohsen Alshehri, and Katabathini Narasimharao. 2022. "Visible Light Active Magnesium Silicate–Graphitic Carbon Nitride Nanocomposites for Methylene Blue Degradation and Pb2+ Adsorption" Catalysts 12, no. 10: 1256. https://doi.org/10.3390/catal12101256
APA StyleAlnassar, M. A., Alshehri, A., & Narasimharao, K. (2022). Visible Light Active Magnesium Silicate–Graphitic Carbon Nitride Nanocomposites for Methylene Blue Degradation and Pb2+ Adsorption. Catalysts, 12(10), 1256. https://doi.org/10.3390/catal12101256