Antimicrobial Activity of Commercial Photocatalytic SaniTise™ Window Glass
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Glass Surfaces
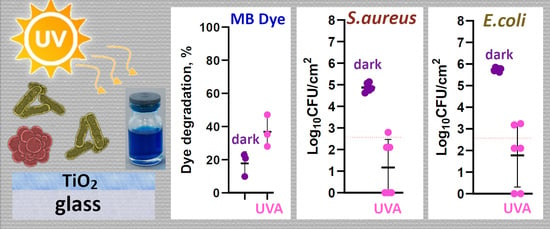
2.2. Photocatalytic Activity of Glass Surfaces
2.3. Antibacterial Effect of Glass Surfaces
3. Materials and Methods
3.1. Glass Materials
3.2. Characterization of the Glass Surfaces
3.3. Evaluation of Antibacterial Efficacy of TiO2 Coated Glass Surfaces
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padmanabhan, N.T.; Honey, J. Titanium dioxide based self-cleaning smart surfaces: A short review. J. Environ. Chem. Eng. 2020, 8, 104211. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Gamage, J.; Zhang, Z. Applications of Photocatalytic Disinfection. Int. J. Photoenergy 2010, 764870. [Google Scholar] [CrossRef] [Green Version]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- De Castillo, C.D.; Correa, M.G.; Martinez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance—A One Health Perspective; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.; Fernández-García, M.; et al. Understanding the Antimicrobial Mechanism of TiO2-Based Nanocomposite Films in a Pathogenic Bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [Green Version]
- Kiwi, J.; Nadtochenko, V. Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopy. Langmuir 2005, 21, 4631–4641. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Williamson, B.A.D.; Sathasivam, S.; Kafizas, A.; Alqahtani, M.; Sotelo-Vazquez, C.; Buckeridge, J.; Wu, J.; Nair, S.P.; Scanlon, D.O.; et al. Enhanced Photocatalytic and Antibacterial Ability of Cu-Doped Anatase TiO2 Thin Films: Theory and Experiment. ACS Appl. Mater. Interfaces 2020, 12, 15348–15361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushnie, T.P.T.; Robertson, P.K.J.; Officer, S.; Pollard, P.M.; Prabhu, R.; Mccullagh, C.; Robertson, J.M.C. Photobactericidal effects of TiO2 thin films at low temperatures—A preliminary study. J. Photochem. Photobiol. A Chem. 2010, 216, 290–294. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Mills, A.; Lepre, A.; Elliott, N.; Bhopal, S.; Parkin, I.P.; O’Neill, S.A. Characterisation of the photocatalyst Pilkington Activ™: A reference film photocatalyst? J. Photochem. Photobiol. A Chem. 2003, 160, 213–224. [Google Scholar] [CrossRef]
- Garlisi, C.; Palmisano, G. Radiation-free superhydrophilic and antifogging properties of e-beam evaporated TiO2 films on glass. Appl. Surf. Sci. 2017, 420, 83–93. [Google Scholar] [CrossRef]
- Dunnill, C.W.; Aiken, Z.A.; Kafizas, A.; Pratten, J.; Wilson, M.; Morgan, D.J.; Parkin, I.P. White light induced photocatalytic activity of sulfur-doped TiO2 thin films and their potential for antibacterial application. J. Mater. Chem. 2009, 19, 8747. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.C.; Tang, H.Y.; Yu, J.; Chan, H.C.; Zhang, L.; Xie, Y.; Wang, H.; Wong, S.P. Bactericidal and photocatalytic activities of TiO2 thin films prepared by sol–gel and reverse micelle methods. J. Photochem. Photobiol. A Chem. 2002, 153, 211–219. [Google Scholar] [CrossRef]
- Nippon Sheet Glass Co. Ltd. Pilkington SaniTise™ Brochures. Available online: https://www.pilkington.com/en/global/products/product-categories/health-applications/pilkington-sanitise#brochures (accessed on 23 December 2021).
- Nippon Sheet Glass Co. Ltd. Pilkington SaniTise™ Technical Bulletin. Available online: https://assetmanager-ws.pilkington.com/fileserver.aspx?cmd=get_file&ref=GL112&cd=cd (accessed on 23 December 2021).
- Peruchon, L.; Puzenat, E.; Girard-Egrot, A.; Blum, L.; Herrmann, J.M.; Guillard, C. Characterization of self-cleaning glasses using Langmuir–Blodgett technique to control thickness of stearic acid multilayers. J. Photochem. Photobiol. A Chem. 2008, 197, 170–176. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. NIST Electron Inelastic-Mean-Free-Path Database, Version 1.2, SRD 71; National Institute of Standards and Technology: Gaithersubrg, MD, USA, 2010. Available online: https://www.nist.gov/system/files/documents/srd/SRD71UsersGuideV1-2.pdf (accessed on 31 January 2022).
- Pärna, R.; Joost, U.; Nõmmiste, E.; Käämbre, T.; Kikas, A.; Kuusik, I.; Hirsimäki, M.; Kink, I.; Kisand, V. Effect of cobalt doping and annealing on properties of titania thin films prepared by sol–gel process. Appl. Surf. Sci. 2011, 257, 6897–6907. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Zhu, K.-R.; Zhang, M.-S.; Chen, Q.; Yin, Z. Size and phonon-confinement effects on low-frequency Raman mode of anatase TiO2 nanocrystal. Phys. Lett. A 2005, 340, 220–227. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Huang, P.J. Thermo-Raman studies on anatase and rutile. J. Raman Spectrosc. 1998, 29, 97–102. [Google Scholar] [CrossRef]
- ISO 10678:2010; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. International Organization for Standardization: Geneva, Switzerland, 2010.
- Nippon Sheet Glass Co. Ltd. Ten Top Tips for Installing Self-Cleaning Glass. Available online: https://www.pilkington.com/en-gb/uk/news-insights/featured-articles/ten-top-tips-for-installing-self-cleaning-glass-not-published (accessed on 23 December 2021).
- Kaishu, G. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coat. Technol. 2005, 191, 155–160. [Google Scholar]
- ISO 27447:2019; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Antibacterial Activity of Semiconducting Photocatalytic Materials. International Organization for Standardization: Geneva, Switzerland, 2019.
- Maiorov, V.A. Self-Cleaning Glass. Glass Phys. Chem. 2019, 45, 161–174. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Van Oss, C.J. Acid–base interfacial interactions in aqueous media. Colloids Surf. A Physicochem. Eng. 1993, 78, 1–49. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Shah, K.W.; Li, W. A Review on Catalytic Nanomaterials for Volatile Organic Compounds VOC Removal and Their Applications for Healthy Buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisand, V.; Visnapuu, M.; Rosenberg, M.; Danilian, D.; Vlassov, S.; Kook, M.; Lange, S.; Pärna, R.; Ivask, A. Antimicrobial Activity of Commercial Photocatalytic SaniTise™ Window Glass. Catalysts 2022, 12, 197. https://doi.org/10.3390/catal12020197
Kisand V, Visnapuu M, Rosenberg M, Danilian D, Vlassov S, Kook M, Lange S, Pärna R, Ivask A. Antimicrobial Activity of Commercial Photocatalytic SaniTise™ Window Glass. Catalysts. 2022; 12(2):197. https://doi.org/10.3390/catal12020197
Chicago/Turabian StyleKisand, Vambola, Meeri Visnapuu, Merilin Rosenberg, Dmytro Danilian, Sergei Vlassov, Mati Kook, Sven Lange, Rainer Pärna, and Angela Ivask. 2022. "Antimicrobial Activity of Commercial Photocatalytic SaniTise™ Window Glass" Catalysts 12, no. 2: 197. https://doi.org/10.3390/catal12020197
APA StyleKisand, V., Visnapuu, M., Rosenberg, M., Danilian, D., Vlassov, S., Kook, M., Lange, S., Pärna, R., & Ivask, A. (2022). Antimicrobial Activity of Commercial Photocatalytic SaniTise™ Window Glass. Catalysts, 12(2), 197. https://doi.org/10.3390/catal12020197