Fabrication of Pt-Loaded Catalysts Supported on the Functionalized Pyrolytic Activated Carbon Derived from Waste Tires for the High Performance Dehydrogenation of Methylcyclohexane and Hydrogen Production
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Instruments
2.3. Preparation of the PTC
2.4. Preparation of the Functionalized PTC Supports
2.5. Preparation of the Pt-Based Catalysts
2.6. Catalytic Reaction
2.7. Recycling Assays
3. Results and Discussion
3.1. Characterizations of the Functional PTC Supports
3.2. Characterizations of the Pt-Based Catalysts over the Functional PTC Supports
3.3. Optimization of Dehydrogenation Reaction Conditions
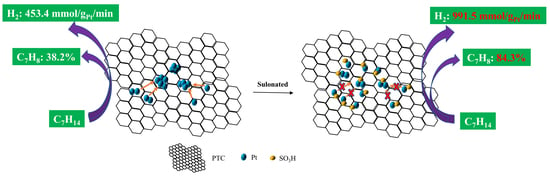
3.4. Dehydrogenation of MCH by the Pt-Based Catalysts over the Functional PTC Supports
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lee, H.; Kim, A.; Lee, B.; Lim, H. Comparative numerical analysis for an efficient hydrogen production via a steam methane reforming with a packed-bed reactor, a membrane reactor, and a sorption-enhanced membrane reactor. Energy Convers. Manag. 2020, 213, 112839. [Google Scholar] [CrossRef]
- Burhan, M.; Shahzad, M.W.; Oh, S.J.; Ng, K.C. A pathway for sustainable conversion of sunlight to hydrogen using proposed compact CPV system. Energy Convers. Manag. 2018, 165, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yao, Z.H.; Haidry, A.A.; Plecenik, T.; Grancic, B.; Roch, T.; Gregor, M.; Plecenik, A. The effect of Nb doping on hydrogen gas sensing properties of capacitor-like Pt/Nb-TiO2/Pt hydrogen gas sensors. J. Alloys Compd. 2019, 806, 1052–1059. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mehrpooya, M. Techno-economic analysis of hydrogen production by solid oxide electrolyzer coupled with dish collector. Energy Convers. Manag. 2018, 173, 167–178. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int. J. Hydrogen Energy 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Kariya, N.; Fukuoka, A.; Ichikawa, M. Efficient evolution of hydrogen from liquid cycloalkanes over Pt-containing catalysts supported on active carbons under “wet–dry multiphase conditions”. Appl. Catal. A 2002, 233, 91–102. [Google Scholar] [CrossRef]
- Kou, Z.; Zhi, Z.; Xu, G.; An, Y.; He, C. Investigation of the performance and deactivation behavior of Raney-Ni catalyst in continuous dehydrogenation of cyclohexane under multiphase reaction conditions. Appl. Catal. A 2013, 467, 196–201. [Google Scholar] [CrossRef]
- Li, J.F.; Chai, Y.M.; Liu, B.; Wu, Y.L.; Li, X.H.; Tang, Z.; Liu, Y.Q.; Liu, C.G. The catalytic performance of Ni2P/Al2O3 catalyst in comparison with Ni/Al2O3 catalyst in dehydrogenation of cyclohexane. Appl. Catal. A 2014, 469, 434–441. [Google Scholar] [CrossRef]
- Zahid, A.H.; Amin, N.; Nisar, F.; Saghir, S. Analysis of MTH-System (Methylcyclohexane-Toluene-Hydrogen-System) for hydrogen production as fuel for power plants. Int. J. Hydrogen Energy 2020, 45, 32234–32242. [Google Scholar] [CrossRef]
- Shukla, A.A.; Gosavi, P.V.; Pande, J.V.; Kumar, V.P.; Chary, K.V.R.; Biniwale, R.B. Efficient hydrogen supply through catalytic dehydrogenation of methylcyclohexane over Pt/metal oxide catalysts. Int. J. Hydrogen Energy 2010, 35, 4020–4026. [Google Scholar] [CrossRef]
- Miao, L.; Yan, J.; Wang, W.Y.; Huang, Y.P.; Li, W.S.; Yang, Y.Q. Dehydrogenation of methylcyclohexane over Pt supported on Mg-Al mixed oxides catalyst: The effect of promoter Ir. Chin. J. Chem. Eng. 2020, 28, 2337–2342. [Google Scholar] [CrossRef]
- Sugiura, Y.; Nagatsuka, T.; Kubo, K.; Hirano, Y.; Nakamura, A.; Miyazawa, K.; Iizuka, Y.; Furuta, S.; Iki, H.; Higo, T.; et al. Dehydrogenation of Methylcyclohexane over Pt/TiO2-Al2O3 Catalysts. Chem. Lett. 2017, 46, 1601–1604. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, S.; Fang, T. Enhancing the catalytic activity and selectivity of PdAu/SiO2 bimetallic catalysts for dodecahydro-N-ethylcarbazole dehydrogenation by controlling the particle size and dispersion. ACS Appl. Energy Mater. 2019, 2, 7233–7243. [Google Scholar] [CrossRef]
- Copéret, C.; Adolfsson, H.; Sharpless, K.B. A simple and efficient method for epoxidation of terminal alkenes. Chem. Commun. 1997, 16, 1565–1566. [Google Scholar] [CrossRef]
- Thomas, B.S.; Gupta, R.C.; Panicker, V.J. Recycling of waste tire rubber as aggregate in concrete: Durability-related performance. J. Clean. Prod. 2016, 112, 504–513. [Google Scholar] [CrossRef]
- Brandsma, S.H.; Brits, M.; Groenewoud, Q.R.; Velzen, M.J.M.; Leonards, P.E.G.; Boert, J. Chlorinated Paraffins in Car tires recycled to rubber granulates and play-ground tiles. Environ. Sci. Technol. 2019, 53, 7595–7603. [Google Scholar] [CrossRef] [Green Version]
- Darmstadt, H.; Roy, C.; Kaliaguine, S. ESCA characterization of commercial carbon blacks and carbon blacks from vacuum pyrolysis of used tires. Carbon 1994, 32, 1399–1405. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, J.Y.; Ko, Y.K.; Reucroft, P.J.; Zondlo, J.W. Surface analysis of carbon black waste materials from tire residues. Appl. Surf. Sci. 1999, 141, 107–113. [Google Scholar] [CrossRef]
- Makrigianni, V.; Giannakas, A.; Deligiannakis, Y.; Konstantinou, I. Adsorption of phenol and methylene blue from aqueous solutions by pyrolytic tire char: Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2015, 3, 574–582. [Google Scholar] [CrossRef]
- Salehs, T.A.; Al-Saadi, A.A.; Gupta, V.K. Carbonaceous adsorbent prepared from waste tires: Experimental and computational evaluations of organic dye methyl orange. J. Mol. Liq. 2014, 191, 85–91. [Google Scholar] [CrossRef]
- Esfandbod, M.; Merritt, C.R.; Rashti, M.R.; Singh, B.; Boyd, S.E.; Srivastava, P.; Brown, C.L.; Butler, O.M.; Kookana, R.S.; Chen, C. Role of oxygen-containing functional groups in forest fire-generated and pyrolytic chars for immobilization of copper and nickel. Environ. Pollut. 2017, 220, 946–954. [Google Scholar] [CrossRef]
- Han, Y.; Dong, X.T.; Zhang, C.; Liu, S.X. Hierarchical porous carbon hollow-spheres as a high performance electrical double-layer capacitor material. J. Power Sources 2012, 211, 92–96. [Google Scholar] [CrossRef]
- Han, Y.; Dong, X.T.; Zhang, C.; Liu, S.X. Easy synthesis of honeycomb hierarchical porous carbon and its capacitive performance. J. Power Sources 2013, 227, 118–122. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, P.P.; Dong, X.T.; Zhang, C.; Liu, S.X. Improvement in electrochemical capacitance of activated carbon from scrap tires by nitric acid treatment. Front. Mater. Sci. 2014, 8, 391–398. [Google Scholar] [CrossRef]
- Ji, R.N.; Yu, K.; Lou, L.L.; Zhang, C.; Han, Y.; Pan, S.; Liu, S.X. Chiral Mn (III) salen complexes immobilized directly on pyrolytic waste tire char for asymmetric epoxidation of unfunctionalized olefins. Inorg. Chem. Commun. 2012, 25, 65–69. [Google Scholar] [CrossRef]
- Simaioforidou, A.; Papastergiou, M.; Margellou, A.; Petrakis, D.; Louloudi, M. Activated vs. pyrolytic carbon as support matrix for chemical functionalization: Efficient heterogeneous non-Heme Mn (II) catalysts for alkene oxidation with H2O2. J. Mol. Catal. A Chem. 2017, 426, 516–525. [Google Scholar] [CrossRef]
- Seristatidou, E.; Mavrogiorgou, A.; Konstantinou, I.; Louloudi, M.; Deligiannakis, Y. Recycled carbon (RC) materials made functional: An efficient heterogeneous Mn-RC catalyst. J. Mol. Catal. A Chem. 2015, 403, 84–92. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, X.Q.; Liu, S.X. Hydrogen production by catalytic dehydrogenation of methylcyclohexane over Pt catalysts supported on pyrolytic waste tire char. Int. J. Hydrogen Energy 2011, 36, 8902–8907. [Google Scholar] [CrossRef]
- Mavrogiorgou, A.; Simaioforidou, A.; Louloudi, M. Pyrolytic carbon as support matrix for heterogeneous oxidation catalysts: The influence of pyrolytic process on catalytic behavior. J. Environ. Chem. Eng. 2018, 6, 1127–1136. [Google Scholar] [CrossRef]
- Ye, H.L.; Liu, S.X.; Zhang, C.; Cai, Y.Q.; Shi, Y.F. Dehydrogenation of methylcyclohexane over Pt-based catalysts supported on functional granular activated carbon. RSC Adv. 2021, 11, 29287–29297. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; RamiroBelo, C.; Mourão, P.A. Pesticides abatement using activated carbon produced from a mixture of synthetic polymers by chemical activation with KOH and K2CO3. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100261. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Q.; Wang, F.M.; Zhan, X.B.; Sun, M.S.; Hu, J.Q.; Zhai, Y.; Lv, H.H.; Lv, G.J. Highly dispersed Pd nanoparticles supported on γ-Al2O3 modified by minimal 3-aminopropyltriethoxysilane as effective catalysts for 2-ethyl-anthraquinone hydrogenation. Appl. Catal. A 2021, 619, 118124. [Google Scholar] [CrossRef]
- Feng, J.T.; Wang, H.Y.; Evans, D.G.; Duan, X.; Li, D.Q. Catalytic hydrogenation of ethylanthraquinone over highly dispersed eggshell Pd/SiO2–Al2O3 spherical catalysts. Appl. Catal. A 2010, 382, 240–245. [Google Scholar] [CrossRef]
- Ma, Q.Q.; Wang, N.; Liu, G.Z.; Wang, L. Enhanced performance of Pd nanoparticles on SBA-15 grafted with alkyltrialkoxysilane in 2-ethyl-anthraquinone hydrogenation. Microporous Mesoporous Mater. 2019, 279, 245–251. [Google Scholar] [CrossRef]
- Qi, B.; Wang, Y.B.; Lou, L.L.; Huang, L.Y.; Yang, Y.; Liu, S.X. Solvent-free aerobic oxidation of alcohols over palladium supported on MCM-41. J. Mol. Catal. A Chem. 2013, 370, 95–103. [Google Scholar] [CrossRef]
- Iost, K.N.; Borisov, V.A.; Temerev, V.L.; Surovikin, Y.V.; Pavluchenko, P.E.; Trenikhin, M.V.; Arbuzov, A.B.; Shlyapin, D.A.; Tsyrulnikov, P.G.; Vedyagin, A.A. Mechanism of Pt interfacial interaction with carbonaceous support under reductive conditions. React. Kinet. Mech. Catal. 2019, 127, 103–115. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Sun, X.T.; Wang, S.Y.; Peng, F.; Bao, F.; Su, Y.Y.; Li, Y.Y.; Lee, S.T.; He, Y. Facile, large-quantity synthesis of stable, tunable-color silicon nanoparticles and their application for long-term cellular imaging. ACS Nano 2015, 9, 5958–5967. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, L.; Li, B.; Song, Y.; Zhao, X.; Zhang, G.; Zhang, S.; Lu, S.; Zhang, J.; Wang, H.; et al. Investigation of photoluminescence mechanism of graphene quantum dots and evaluation of their assembly into polymer dots. Carbon 2014, 77, 462–472. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Masteri-Farahani, M.; Shahsavarifar, S. Chemical modification of re duce d graphene oxide with sulfonic acid groups: Efficient solid acids for acetalization and esterification reactions. J. Taiwan Inst. Chem. Eng. 2019, 102, 34–43. [Google Scholar] [CrossRef]
- Pyanova, L.G.; Luzyanina, L.S.; Drozdov, V.A.; Veselovskaya, A.V.; Arbuzov, A.B.; Likholobov, V.A. Study of the effect of a number of oxidizers on variation of composition of surface functional groups, porous structure, and adsorption properties of composite carbon-carbon sorbent. Prot. Met. Phys. Chem. Surf. 2010, 46, 320–324. [Google Scholar] [CrossRef]
- Tang, X.D.; Yu, H.M.; Bui, B.; Wang, L.Y.; Xing, C.; Wang, S.Y.; Chen, M.L.; Hu, Z.Z.; Chen, W. Nitrogen-doped fluorescence carbon dots as multi-mechanism detection for iodide and curcumin in biological and food samples. Bioact. Mater. 2021, 6, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Sandt, C.; Waeytens, J.; Deniset-Besseau, A.; Nielsen-Leroux, C.; Réjassed, A. Use and misuse of FTIR spectroscopy for studying the bio-oxidation of plastics. Spectrochim. Acta Part A 2021, 258, 119841. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.X.; Rowland, C.E.; Schaller, R.D.; Korgel, B.A. Synthesis and ligand exchange of thiol-capped silicon nanocrystals. Langmuir 2015, 31, 6886–6893. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.; Zhang, H.; Kim, Y.K.; Harbottle, D.; Lee, J.W. Enhanced adsorption capacity and selectivity towards strontium ions in aqueous systems by sulfonation of Co2 derived porous carbon. RSC Adv. 2017, 7, 54546–545537. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Jin, Y.; Li, Z.; Peng, F.; Wang, H. Synthesis and characterization of sulfonated single-walled carbon nanotubes and their performance as solid acid catalyst. J. Solid State Chem. 2008, 181, 432–438. [Google Scholar] [CrossRef]
- Machado, J.; Castanheiro, J.E.; Matos, I.; Ramos, A.M.; Vital, J.; Fonseca, I.M. SBA-15 with sulfonic acid groups as a Green Catalyst for the acetoxylation of α-pinene. Microporous Mesoporous Mater. 2012, 163, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.P.; Xie, Y.P.; Chen, W.; Xia, M.W.; Li, K.X.; Chen, Z.Q.; Chen, Y.Q.; Yang, H.P. Investigation on co-pyrolysis of lignocellulosic biomass and amino acids using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 196, 320–329. [Google Scholar] [CrossRef]
- Viscardia, R.; Barbarossa, V.; Maggi, R.; Pancrazzi, F. Effect of acidic MCM-41 mesoporous silica functionalized with sulfonic acid groups catalyst in conversion of methanol to dimethyl. Energy Rep. 2020, 6, 49–55. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Ye, H.L.; Shang, Y.; Wang, H.Y.; Ma, Y.L.; He, X.W.; Li, W.Y.; Li, Y.H.; Zhang, Y.K. Determination of Fe(Ⅲ) ion and cellular bioimaging based on a novel photoluminescent silicon nanoparticles. Talanta 2021, 230, 122294. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.M.; Xu, G.T. Peak overlaps and corresponding solutions in theX-ray photoelectron spectroscopic study of hydrodesulfurization catalysts. Appl. Surf. Sci. 2010, 256, 3413–3417. [Google Scholar] [CrossRef]
- Qiu, L.M.; Zou, K.; Xu, G.T. Investigation on the sulfur state and phase transformation of spent and regenerated S zorb sorbents using XPS and XRD. Appl. Surf. Sci. 2013, 266, 230–234. [Google Scholar] [CrossRef]
- Bittencourt, C.; Hecq, M.; Felten, A.; Pireaux, J.J.; Ghijsen, J.; Felicissimo, M.P.; Rudolf, P.; Drube, W.; Ke, X.; Van, T.G. Platinum-carbon nanotube interaction. Chem. Phys. Lett. 2008, 462, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.T.; Huang, Y.P.; Mi, C.J.; Wu, K.; Wang, W.Y.; Li, W.S.; Yang, Y.Q. Density functional theory study on catalytic dehydrogenation of methylcyclohexane on Pt (111). Int. J. Hydrogen Energy 2020, 45, 6727–6737. [Google Scholar] [CrossRef]
- Miller, J.T.; Koningsberger, D.C. The origin of sulfur tolerance in supported platinum catalysts: The relationship between structural and catalytic properties in acidic and alkaline Pt/LTL. J. Catal. 1996, 162, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Lee, K.T.; Holden, R.; Zhang, L.; Zhang, J.; Botton, G.A.; Couillard, M.; Nazar, L.F. Nanocrystalline intermetallics on mesoporous carbon for direct formic acid fuel cell anodes. Nat. Chem. 2010, 2, 286–293. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.X.; Yan, Q.Q.; Xu, S.L.; Chu, S.Q.; Chen, P.; Lin, Y.; Liang, H.W. A sulfur-tethering synthesis strategy toward high-loading atomically dispersed noble metal catalysts. Sci. Adv. 2019, 5, eaax6322. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Luo, X.; Ma, Y.F.; Chu, S.Q.; Chen, S.; Zheng, X.S.; Lu, J.L.; Wu, X.J.; Liang, H.W. Sulfur stabilizing metal nanoclusters on carbon at high temperatures. Nat. Chem. 2021, 12, 3135. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, W.; Li, X.; Tan, D.; Han, X.; Bao, X. One-pot encapsulation of Pt nanoparticles into the mesochannels of SBA-15 and their catalytic dehydrogenation of methylcyclohexane. Catal. Lett. 2007, 119, 159–164. [Google Scholar] [CrossRef]
- Wang, W.Y.; Miao, L.; Wu, K.; Chen, G.L.; Huang, Y.P.; Yang, Y.Q. Hydrogen evolution in the dehydrogenation of methylcyclohexane over Pt/Ce-Mg-Al-O catalysts derived from their layered double hydroxides. Int. J. Hydrogen Energy 2019, 44, 2918–2925. [Google Scholar] [CrossRef]
- Yang, X.; Song, Y.; Cao, T.T.; Wang, L.; Song, H.T.; Lin, W. The double tuning effect of TiO2 on Pt catalyzed dehydrogenation of methylcyclohexane. Mol. Catal. 2020, 492, 110971. [Google Scholar] [CrossRef]
- Li, X.Y.; Ma, D.; Bao, X.H. Dispersion of Pt catalysts supported on activated carbon and their catalytic performance in methylcyclohexane dehydrogenation. Chin. J. Catal. 2008, 29, 259–263. [Google Scholar] [CrossRef]






| Supports | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| PTC | 902 | 1.071 | 4.75 |
| PTC-S | 898 | 1.133 | 5.05 |
| PTC-K | 906 | 1.160 | 5.12 |
| PTC-NH | 490 | 0.833 | 6.80 |
| Catalysts | Adsorption Capacity of CO (μL/g Catalysts) | Surface Area of Metal (m2/g Sample) | Dispersion of Active Component Pt (%) | Mean Size of Pt (nm) |
|---|---|---|---|---|
| Pt/PTC | 58.9 | 63.3 | 25.6 | 4.42 |
| Pt/PTC-S | 122.7 | 131.9 | 53.4 | 2.12 |
| Pt/PTC-K | 28.0 | 30.1 | 12.2 | 9.31 |
| Pt/PTC-NH | 9.89 | 10.6 | 4.3 | 26.3 |
| Catalysts | Temperature (°C) | Rate of Liquid MCH (mL/min) | Catalyst Weight | Pt loading Content (wt %) | Conversion of MCH (%) | H2 Evolution Rate (mmol/gPt/min) | Refs. |
|---|---|---|---|---|---|---|---|
| Pt/SBA-15 | 300 °C | 0.03 | 0.05 | 3 | 65 (initial) | 308.6 | [60] |
| Pt/Ce-Mg-Al O | 300 °C | - | - | 0.35 | 49.8 | 686.9 | [61] |
| Pt/coconut activated carbon | 300 °C | 0.03 | 0.03 | 1 | 42 | 598.2 | [62] |
| Pt/pyrolytic waste activated carbon | 300 °C | 0.03 | 0.554 | 0.4 | 95 | 305.3 | [29] |
| Pt/GAC-S | 300 °C | 0.03 | 0.3 | 0.2 | 63 | 741.1 | [31] |
| PtSn-5/Mg-Al-O-350 | 300 °C | 0.1 | 0.5 | 2 | 90.5 | 214.8 | [63] |
| Pt/PTC-S | 300 °C | 0.03 | 0.3 | 0.2 | 84.3 | 991.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, H.; Wang, T.; Liu, S.; Zhang, C.; Cai, Y. Fabrication of Pt-Loaded Catalysts Supported on the Functionalized Pyrolytic Activated Carbon Derived from Waste Tires for the High Performance Dehydrogenation of Methylcyclohexane and Hydrogen Production. Catalysts 2022, 12, 211. https://doi.org/10.3390/catal12020211
Ye H, Wang T, Liu S, Zhang C, Cai Y. Fabrication of Pt-Loaded Catalysts Supported on the Functionalized Pyrolytic Activated Carbon Derived from Waste Tires for the High Performance Dehydrogenation of Methylcyclohexane and Hydrogen Production. Catalysts. 2022; 12(2):211. https://doi.org/10.3390/catal12020211
Chicago/Turabian StyleYe, Hongli, Tianci Wang, Shuangxi Liu, Cui Zhang, and Youqiong Cai. 2022. "Fabrication of Pt-Loaded Catalysts Supported on the Functionalized Pyrolytic Activated Carbon Derived from Waste Tires for the High Performance Dehydrogenation of Methylcyclohexane and Hydrogen Production" Catalysts 12, no. 2: 211. https://doi.org/10.3390/catal12020211
APA StyleYe, H., Wang, T., Liu, S., Zhang, C., & Cai, Y. (2022). Fabrication of Pt-Loaded Catalysts Supported on the Functionalized Pyrolytic Activated Carbon Derived from Waste Tires for the High Performance Dehydrogenation of Methylcyclohexane and Hydrogen Production. Catalysts, 12(2), 211. https://doi.org/10.3390/catal12020211