Dispersion and Stabilization of Supported Layered Double Hydroxide-Based Nanocomposites on V-Based Catalysts for Nonoxidative Dehydrogenation of Isobutane to Isobutene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Composite Oxide Supports and Catalysts
2.1.1. Phase Composition and Textural Characteristics of Catalysts
2.1.2. Polymerization Degree of VOx Species
2.1.3. Surface Acidic Properties of Catalysts
2.1.4. Reducibility and Surface Chemical State of VOx Species
2.2. Coking Behavior on the Used Catalysts
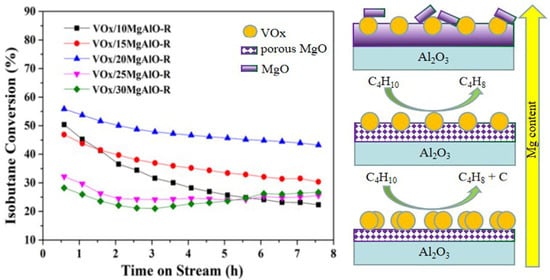
2.3. Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Composite Oxide Support Preparation
3.3. Catalyst Preparation
3.4. Catalyst Characterization
3.5. Catalytic Performance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sattler, J.J.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.L.; Li, P.H.; Zhao, F.; Song, H.L.; Xia, C.G. Selective aromatization of biomass derived diisobutylene to p-xylene over supported non-noble metal catalysts. Catal. Today 2016, 276, 105–111. [Google Scholar] [CrossRef]
- Xie, Y.; Luo, R.; Sun, G.; Chen, S.; Zhao, Z.J.; Mu, R.; Gong, J. Facilitating the reduction of V-O bonds on VOx/ZrO2 catalysts for non-oxidative propane dehydrogenation. Chem. Sci. 2020, 11, 3845–3851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Zhang, H.; Zhang, S.; Wang, G.; Zhu, X.; Li, C. Dehydrogenation of isobutane over a Ni–P/SiO2 catalyst: Effect of P addition. Ind. Eng. Chem. Res. 2019, 58, 7834–7843. [Google Scholar] [CrossRef]
- Chen, C.; Sun, M.L.; Hu, Z.P.; Ren, J.T.; Zhang, S.M.; Yuan, Z.Y. New insight into the enhanced catalytic performance of ZnPt/HZSM-5 catalysts for direct dehydrogenation of propane to propylene. Catal. Sci. Technol. 2019, 9, 1979–1988. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Zhou, H.; Wang, L.; Wang, L.; Zhu, Q.; Xiao, J.; Meng, X.; Chen, J.; Xiao, F.S. Coking-resistant iron catalyst in ethane dehydrogenation achieved through siliceous zeolite modulation. J. Am. Chem. Soc. 2020, 142, 16429–16436. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.M.; Wang, Z.; Yuan, Z.Y. Ultrasmall Co confined in the silanols of dealuminated beta zeolite: A highly active and selective catalyst for direct dehydrogenation of propane to propylene. J. Catal. 2020, 383, 77–87. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, L.; Zhao, Z.J.; Tian, H.; Wu, T.F.; Gong, J.L. Platinum-modified ZnO/Al2O3 for propane dehydrogenation: Minimized platinum usage and improved catalytic stability. ACS Catal. 2016, 6, 2158–2162. [Google Scholar] [CrossRef]
- Sokolov, S.; Stoyanova, M.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. Comparative study of propane dehydrogenation over V-, Cr-, and Pt-based catalysts: Time on-stream behavior and origins of deactivation. J. Catal. 2012, 293, 67–75. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, H.J.; Yang, M.M.; Ma, Z.P.; Zhao, L.M.; Xing, W.; Wu, P.P.; Liu, X.M.; Mintova, E.L.N.; Bai, P.; et al. Highly stable phosphine modified VOx/Al2O3 catalyst in propane dehydrogenation. Appl. Catal. 2020, 274, 119809. [Google Scholar] [CrossRef]
- Bai, P.; Ma, Z.; Li, T.; Tian, Y.; Zhang, Z.; Zhong, Z.; Xing, W.; Wu, P.; Liu, X.; Yan, Z. Relationship between surface chemistry and catalytic performance of mesoporous γ-Al2O3 supported VOx catalyst in catalytic dehydrogenation of propane. ACS Appl. Mater. Interfaces 2016, 8, 25979–25990. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Fan, X.; Liu, F.; Wang, L.; Lin, H.; Li, Y.; Lee, S.T. Single vanadium atoms anchored on graphitic carbon nitride as a high-performance catalyst for non-oxidative propane dehydrogenation. ACS Nano 2020, 14, 5772–5779. [Google Scholar] [CrossRef] [PubMed]
- Dasireddy, V.D.B.C.; Singh, S.; Friedrich, H.B. Effect of the Support on the oxidation of heptane using vanadium supported on alkaline earth metal hydroxyapatites. Catal. Lett. 2014, 145, 668–678. [Google Scholar] [CrossRef]
- Dasireddy, V.D.B.C.; Friedrich, H.B.; Singh, S. Studies towards a mechanistic insight into the activation of n-octane using vanadium supported on alkaline earth metal hydroxyapatites. Appl. Catal. A Gen. 2013, 467, 142–153. [Google Scholar] [CrossRef]
- Dasireddy, V.D.B.C.; Singh, S.; Friedrich, H.B. Vanadium oxide supported on non-stoichiometric strontium hydroxyapatite catalysts for the oxidative dehydrogenation of n-octane. J. Mol. Catal. A Chem. 2014, 395, 398–408. [Google Scholar] [CrossRef]
- Tian, Y.P.; Liu, X.M.; Zhan, W.L.; Cheng, S.X.; Zhang, L.L.; Yan, Z.F. Elucidation of active species and reaction mechanism of sulfide V-K/Al2O3 catalyst for isobutane dehydrogenation. Appl. Surf. Sci. 2021, 569, 5772–5779. [Google Scholar] [CrossRef]
- Tian, Y.P.; Liu, X.M.; Mintova, S.; Zhang, L.L.; Pan, Y.Y.; Rives, A.; Liu, Y.A.; Wei, L.; Yan, Z.F. Isobutane dehydrogenation over high-performanced sulfide V-K/γ-Al2O3 catalyst: Modulation of vanadium species and intrinsic effect of potassium. J. Colloid Interface Sci. 2021, 600, 440–448. [Google Scholar] [CrossRef]
- Hu, P.; Lang, W.Z.; Yan, X.; Chu, L.F.; Guo, Y.J. Influence of gelation and calcination temperature on the structure-performance of porous VOX-SiO2 solids in non-oxidative propane dehydrogenation. J. Catal. 2018, 358, 108–117. [Google Scholar] [CrossRef]
- Chen, C.; Sun, M.; Hu, Z.; Liu, Y.; Zhang, S.; Yuan, Z.Y. Nature of active phase of VO catalysts supported on SiBeta for direct dehydrogenation of propane to propylene. Chin. J. Catal. 2020, 41, 276–285. [Google Scholar] [CrossRef]
- Rodemerck, U.; Stoyanova, M.; Kondratenko, E.V.; Linke, D. Influence of the kind of VOx structures in VOx/MCM-41 on activity, selectivity and stability in dehydrogenation of propane and isobutane. J. Catal. 2017, 352, 256–263. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Chesalov, Y.A.; Saraev, A.A.; Tsapina, A.M. A Mechanistic Study of Dehydrogenation of Propane over Vanadia-Titania Catalysts. J. Phys. Chem. C 2019, 123, 19668–19680. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.J.; Wu, T.F.; Zeng, L.; Gong, J.L. Nature of the active sites of VOx/Al2O3 catalysts for propane dehydrogenation. ACS Catal. 2016, 6, 5207–5214. [Google Scholar] [CrossRef]
- Rodemerck, U.; Sokolov, S.; Stoyanova, M.; Bentrup, U.; Linke, D.; Kondratenko, E.V. Influence of support and kind of VO species on isobutene selectivity and coke deposition in non-oxidative dehydrogenation of isobutane. J. Catal. 2016, 338, 174–183. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhou, G.L.; Chen, Z.W.; Li, Q.; Zhou, H.J.; Xu, C.M. Enhancing the vanadium dispersion on V-MCM-41 by boron modification for efficient iso-butane dehydrogenation. Appl. Catal. A 2018, 555, 171–177. [Google Scholar] [CrossRef]
- Wu, T.F.; Liu, G.; Zeng, L.; Sun, G.D.; Chen, S.; Mu, R.T.; Gbonfoun, S.A.; Zhao, Z.J.; Gong, J.L. Structure and catalytic consequence of Mg-modified VOx/Al2O3 catalysts for propane dehydrogenation. AIChE J. 2017, 63, 4911–4919. [Google Scholar] [CrossRef]
- Shan, Y.L.; Zhao, W.T.; Zhao, S.L.; Wang, X.X.; Sun, H.L.; Yu, W.L.; Ding, J.W.; Feng, X.; Chen, D. Effects of alumina phases on the structure and performance of VOx/Al2O3 catalysts in non-oxidative propane dehydrogenation. Mol. Catal. 2021, 504, 111466. [Google Scholar] [CrossRef]
- Wu, Z.; Stair, P. UV Raman spectroscopic studies of V/θ-Al2O3 catalysts in butane dehydrogenation. J. Catal. 2006, 237, 220–229. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Z.; Guo, M.Q.; Li, X.X.; Lin, Y.J.; Zhang, L.H. Effect of reduction atmosphere on structure and catalytic performance of PtIn/Mg(Al)O/ZnO for propane dehydrogenation. Catalysts 2020, 10, 485. [Google Scholar] [CrossRef]
- Li, J.X.; Zhang, M.; Song, Z.; Liu, S.; Wang, J.M.; Zhang, L.H. Hierarchical PtIn/Mg(Al)O derived from reconstructed PtIn-hydrotalcite-like compounds for highly efficient propane dehydrogenation. Catalysts 2019, 9, 767. [Google Scholar] [CrossRef] [Green Version]
- Xia, K.; Lang, W.Z.; Li, P.P.; Long, L.L.; Yan, X.; Guo, Y.J. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O-x catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2016, 284, 1068–1079. [Google Scholar] [CrossRef]
- Zhu, Y.R.; An, Z.; Song, H.Y.; Xiang, X.; Yang, W.J.; He, J. Lattice-confined Sn (IV/II) stabilizing raft-like Pt clusters: High selectivity and durability in propane dehydrogenation. ACS Catal. 2017, 7, 6973–6978. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; Pietschnig, R. Preparation, characterization and catalytic activity of MgO/SiO2 supported vanadium oxide based catalysts. Catal. Lett. 2013, 144, 97–103. [Google Scholar] [CrossRef]
- Tan, C.; Guo, Y.F.; Sun, J.; Li, W.L.; Zhang, J.B.; Zhao, C.W.; Lu, P. Structurally improved MgO adsorbents derived from magnesium oxalate precursor for enhanced CO2 capture. Fuel 2020, 278, 118379. [Google Scholar] [CrossRef]
- Ding, Y.D.; Song, G.; Zhu, X.; Chen, R.; Liao, Q. Synthesizing MgO with a high specific surface for carbon dioxide adsorption. RSC Adv. 2015, 5, 30929–30935. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, W.; Zhang, H.; Bi, S.; Zhang, Q. Hydrothermal–thermal conversion synthesis of hierarchical porous MgO microrods as efficient adsorbents for lead(II) and chromium(VI) removal. RSC Adv. 2014, 4, 30542–30550. [Google Scholar] [CrossRef]
- Zeng, S.B.; Xu, X.L.; Wang, S.K.; Gong, Q.K.; Liu, R.J.; Yu, Y. Sand flower layered double hydroxides synthesized by co-precipitation for CO2 capture: Morphology evolution mechanism, agitation effect and stability. Mater. Chem. Phys. 2013, 140, 159–167. [Google Scholar] [CrossRef]
- Jin, X.; Wang, R.; Zhou, Y.; Lai, J.; Li, J.; Pei, G.; Chen, S.; Wang, X.; Xiang, J.; Zhu, Z.; et al. A comprehensive experimental and first-principles study on magnesium-vanadium oxides. J. Alloys Compd. 2022, 896, 162862. [Google Scholar] [CrossRef]
- Ono, T.; Ogata, N.; Numata, H.; Miyaryo, Y. A study of active sites for alkene and alkane oxidation over Mo and V mixed oxide catalysts using 18O tracer and Raman spectroscopy. Top. Catal. 2001, 15, 2–4. [Google Scholar] [CrossRef]
- Miao, C.L.; Hui, T.L.; Liu, Y.N.; Feng, J.T.; Li, D.Q. Pd/MgAl-LDH nanocatalyst with vacancy-rich sandwich structure: Insight into interfacial effect for selective hydrogenation. J. Catal. 2019, 370, 107–117. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Cherian, M.; Baerns, M. Oxidative dehydrogenation of propane over differently structured vanadia-based catalysts in the presence of O2 and N2O. Catal. Today 2006, 112, 60–63. [Google Scholar] [CrossRef]
- Rajan, N.P.; Rao, G.S.; Putrakumar, B.; Chary, K.V.R. Vapour phase dehydration of glycerol to acrolein over vanadium phosphorous oxide (VPO) catalyst. RSC Adv. 2014, 4, 53419–53428. [Google Scholar] [CrossRef]
- Chen, X.; Ge, M.; Li, Y.; Liu, Y.; Wang, J.; Zhang, L. Fabrication of highly dispersed Pt-based catalysts on γ-Al2O3 supported perovskite nano islands: High durability and tolerance to coke deposition in propane dehydrogenation. Appl. Surf. Sci. 2019, 490, 611–621. [Google Scholar] [CrossRef]
- Wang, J.M.; Song, Z.; Han, M.X.; Li, X.X.; Zhang, L.H. Molybdenum-based catalysts supported on alumina for direct dehydrogenation of isobutane. Mol. Catal. 2021, 511, 111746. [Google Scholar] [CrossRef]
- Li, Y.Y.; Ge, M.; Wang, J.M.; Guo, M.Q.; Liu, F.J.; Han, M.X.; Xu, Y.H.; Zhang, L.H. Dehydrogenation of isobutane to isobutene over a Pt-Cu bimetallic catalyst in the presence of LaAlO3 perovskite. Chin. J. Chem. Eng. 2021, 32, 203–211. [Google Scholar] [CrossRef]
- Tian, Y.P.; Bai, P.; Liu, S.M.; Liu, X.-M.; Yan, Z.F. VOx-K2O/γ-Al2O3 catalyst for nonoxidative dehydrogenation of isobutane. Fuel Process. Technol. 2016, 151, 31–39. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Wu, T.; Xiong, C.; Sun, G.; Mu, R.; Zeng, L.; Gong, J. Hydroxyl-mediated non-oxidative propane dehydrogenation over VOx/γ-Al2O3 catalysts with improved stability. Angew. Chem. Int. Ed. Engl. 2018, 57, 6791–6795. [Google Scholar] [CrossRef]










| Sample | SBET (m2·g−1) | VT (cm3·g−1) | DAP | DP (%) | |||
|---|---|---|---|---|---|---|---|
| (nm) | 2.5–7.5 nm | 7.5–17.0 nm | 17.0–30.0 nm | ||||
| VOx/10MgAlO | 102 | 0.31 | 12.1 | 14.9 | 51.8 | 33.3 | |
| VOx/15MgAlO | 123 | 0.38 | 12.1 | 11.7 | 52.3 | 36.0 | |
| VOx/20MgAlO | 161 | 0.48 | 9.6 | 5.2 | 62.2 | 32.6 | |
| VOx/25MgAlO | 140 | 0.51 | 9.6 | 5.2 | 56.2 | 38.7 | |
| VOx/30MgAlO | 87 | 0.23 | 12.4 | 21.2 | 50.0 | 28.8 | |
| Catalysts | Tmax (°C) | Peak Area Ratio (%) | ||||
|---|---|---|---|---|---|---|
| Peak I | Peak II | Peak III | Peak I | Peak II | Peak III | |
| VOx/10MgAlO | 159 | 237 | 358 | 25 | 50 | 25 |
| VOx/15MgAlO | 158 | 240 | 363 | 21 | 62 | 17 |
| VOx/20MgAlO | 161 | 256 | 395 | 23 | 66 | 11 |
| VOx/25MgAlO | 160 | 247 | 381 | 25 | 68 | 7 |
| VOx/30MgAlO | 161 | 248 | 383 | 27 | 65 | 8 |
| Reduced Catalysts | V3+ (%) | V4+ (%) | V5+ (%) | AOS |
|---|---|---|---|---|
| VOx/10MgAlO-R | 39 | 36 | 25 | 3.86 |
| VOx/15MgAlO-R | 37 | 37 | 26 | 3.89 |
| VOx/20MgAlO-R | 35 | 37 | 28 | 3.93 |
| VOx/25MgAlO-R | 34 | 34 | 32 | 3.98 |
| VOx/30MgAlO-R | 31 | 36 | 33 | 4.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Han, M.; Li, X.; Zhang, X.; Wang, Y.; Xu, Y.; Zhang, L. Dispersion and Stabilization of Supported Layered Double Hydroxide-Based Nanocomposites on V-Based Catalysts for Nonoxidative Dehydrogenation of Isobutane to Isobutene. Catalysts 2022, 12, 382. https://doi.org/10.3390/catal12040382
Liu F, Han M, Li X, Zhang X, Wang Y, Xu Y, Zhang L. Dispersion and Stabilization of Supported Layered Double Hydroxide-Based Nanocomposites on V-Based Catalysts for Nonoxidative Dehydrogenation of Isobutane to Isobutene. Catalysts. 2022; 12(4):382. https://doi.org/10.3390/catal12040382
Chicago/Turabian StyleLiu, Fanji, Mingxun Han, Xiangxiang Li, Xiqing Zhang, Yanting Wang, Yanhong Xu, and Lihong Zhang. 2022. "Dispersion and Stabilization of Supported Layered Double Hydroxide-Based Nanocomposites on V-Based Catalysts for Nonoxidative Dehydrogenation of Isobutane to Isobutene" Catalysts 12, no. 4: 382. https://doi.org/10.3390/catal12040382
APA StyleLiu, F., Han, M., Li, X., Zhang, X., Wang, Y., Xu, Y., & Zhang, L. (2022). Dispersion and Stabilization of Supported Layered Double Hydroxide-Based Nanocomposites on V-Based Catalysts for Nonoxidative Dehydrogenation of Isobutane to Isobutene. Catalysts, 12(4), 382. https://doi.org/10.3390/catal12040382