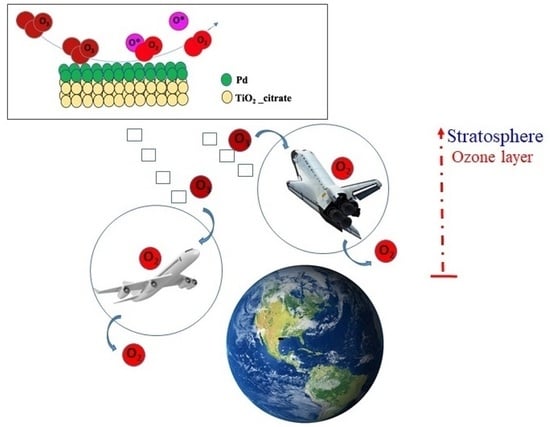
Low-Temperature O3 Decomposition over Pd-TiO2 Hybrid Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Synthesis
2.2. Physicochemical and Morphological Properties of Catalysts
2.3. Catalytic Properties
2.3.1. Effect of Pd Free Support on O3 Decomposition
2.3.2. Catalytic Activity in Dry and Humid Conditions
2.3.3. Effect of the Catalyst Weight in the O3 Decomposition
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Preparation of Hybrid (Cit)1(TiO2)x (x = 20; 50 and 100)
3.2.2. Preparation of TiO2_Citrate Free
3.2.3. Preparation of Pd Supported on Hybrid TiO2
3.3. Characterization Methods
3.4. Catalytic Test
- -
- mPd is the mass of Pd in the catalytic (g);
- -
- Vgas is the total molar flow rate (=1000/(60 × 22,400)) = 7.5 × 10−4 mol·s−1;
- -
- XO3 is conversion of O3;
- -
- YO3 is the mole fraction of O3 in the in-let gas mixture (80% Ar/20% O2);
- -
- MPd is the molecular weight of Pd (106.42 g·mol−1);
- -
- d is the dispersion of the Pd particles determined by the dynamic pulsed hydrogen chemisorption technique.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.-C.; Klemeš, J.J.; Dong, X.; Fan, W.; Xu, Z.; Wang, Y.; Varbanov, P.S. Air pollution terrain nexus: A review considering energy generation and consumption. Renew. Sustain. Energy Rev. 2019, 105, 71–85. [Google Scholar] [CrossRef]
- Touati, T.; Valange, S.; Reinholdt, M.; Batiot-Dupeyrat, C.; Clacens, J.-M.; Tatibouët, J.-M. Low Temperature Catalytic Oxidation of Ethanol Using Ozone over Manganese Oxide-Based Catalysts in Powdered and Monolithic Forms. Catalysts 2022, 12, 172. [Google Scholar] [CrossRef]
- Yue, X.; Unger, N.; Harper, K.; Xia, X.G.; Liao, H.; Zhu, T.; Xiao, J.F.; Feng, Z.Z.; Li, J. Ozone and Haze Pollution Weakens Net Primary Productivity in China. Atmos. Chem. Phys. 2017, 17, 6073–6089. [Google Scholar] [CrossRef] [Green Version]
- Fann, N.; Lamson, A.D.; Anenberg, S.C.; Wesson, K.; Risley, D.; Hubbell, B.J. Estimating the National Public Health Burden Associated with Exposure to Ambient PM2.5 and Ozone. Risk Anal. 2012, 32, 81–95. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Anenberg, S.C.; Schwartz, J.; Shindell, D.; Amann, M.; Faluvegi, G.; Klimont, Z.; Janssens-Maenhout, G.; Pozzoli, L.; Dingenen, R.V.; Vignati, E.; et al. Global air quality and health co-benefits of mitigating near-term climate change through methane and black carbon emission controls. Environ. Health Perspect. 2012, 120, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Young, C.J.; Zhou, S.; Siegel, J.A.; Kahan, T.F. Illuminating the dark side of indoor oxidants. Environ. Sci. 2019, 21, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Tatibouët, J.-M.; Valange, S.; Touati, H. Near-ambient temperature ozone decomposition kinetics on manganese oxide-based catalysts. Appl. Catal. A Gen. 2019, 569, 126–133. [Google Scholar] [CrossRef]
- Batakliev, T.; Georgiev, V.; Anachkov, M.; Rakovsky, S.; Zaikov, G.E. Review article: Ozone decomposition. Interdiscip. Toxicol. 2014, 7, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Dhandapani, B.; Oyama, S.T. Review: Gas phase ozone decomposition catalysts. Appl. Catal. B Environ. 1997, 11, 129–166. [Google Scholar] [CrossRef]
- Touati, H.; Guerin, A.; Swesi, Y.; Dupeyrat, C.B.; Philippe, R.; Meille, V.; Clacens, J.-M. Unexpected role of NOx during catalytic ozone abatement at low temperature. Catal. Commun. 2021, 148, 106163. [Google Scholar] [CrossRef]
- Di, Q.; Dai, L.; Wang, Y.; Zanobetti, A.; Choirat, C.; Schwartz, J.D.; Dominici, F. Association of Short-Term Exposure to Air Pollution with Mortality in Older Adults. JAMA 2017, 318, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, M.; Lu, Y.; Zhang, X.; Yang, C. Catalytic removal of ozone and design of an ozone converter for the bleeding air purification of aircraft cabin. Build. Environ. 2017, 115, 25–33. [Google Scholar] [CrossRef]
- Charpin, D.; Pairon, J.-C.; Annesi-Maesano, I.; Caillaud, D.; de Blay, F.; Dixaut, G.; Housset, B.; Meurice, J.-C.; Roussel, I.; Zmirou, D.; et al. La pollution atmosphérique et ses effets sur la santé respiratoire. Document d’experts du groupe pathologies pulmonaires professionnelles environnementales et iatrogéniques (PAPPEI) de la Société de pneumologie de langue française (SPLF). Rev. Mal. Respir. 2016, 33, 484–508. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, G. Comfort and health in commercial aircraft: A literature review. J. R. Soc. Promot. Health 2001, 121, 29–37. [Google Scholar] [CrossRef]
- Baker, E.S.; Barratt, M.R.; Sams, C.F.; Wear, M.L. Human Response to Space Flight. In Principles of Clinical Medicine for Space Flight; Barratt, M., Baker, E., Pool, S., Eds.; Springer: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, P.; Li, J. Photocatalytic degradation of low concentration formaldehyde and simultaneous elimination of ozone by-product using palladium modified TiO2 films under UV254+185nm irradiation. Appl. Catal. B Environ. 2011, 105, 220–228. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, P.; Li, J. Simultaneous Elimination of Formaldehyde and Ozone Byproduct Using Noble Metal Modified TiO2 Films in the Gaseous VUV. Int. J. Photoenergy 2012, 2012, 8. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, M.; Ndour, M.; Ka, O.; D’anna, B.; George, C. Photochemistry of Atmospheric Dust: Ozone Decomposition on Illuminated Titanium Dioxide. Environ. Sci. Technol. 2009, 43, 7437–7442. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; He, H. Transition metal doped cryptomelane-type manganese oxide catalysts for ozone decomposition. Appl. Catal. B Environ. 2017, 201, 503–510. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, J.; Li, K.; Huang, H.; Shrestha, R.P.; Thi, N.; Oanh, K.; Winijkul, E.; Deng, J. Activated carbon supported MnO nanoparticles for efficient ozone decomposition at room temperature. Catal. Today 2019, 335, 573–579. [Google Scholar] [CrossRef]
- Shen, T.; Zhang, X.; Yi, K.; Lin, A.; Tong, S. Solid base Mg-doped ZnO for heterogeneous catalytic ozonation of isoniazid: Performance and mechanism. Sci. Total Environ. 2019, 703, 134983. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J.; Fijołek, L. Catalytic ozonation-Effect of carbon contaminants on the process of ozone decomposition. Appl. Catal. B Environ. 2013, 142–143, 307–314. [Google Scholar] [CrossRef]
- Swesi, Y.; Gillet, A.; Guérin, A.; Zanota, M.-L.; Bornette, F.; Philippe, R.; Meille, V. Comparison of Structured Reactors for Ozone Abatement in Aircrafts at Low Temperature. Ind. Eng. Chem. Res. 2021, 60, 16739–16746. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, W.; Si, W.; Chen, J.; Peng, Y.; Li, J. A novel γ-like MnO2 catalyst for ozone decomposition in high humidity conditions. J. Hazar. Mater. 2021, 420, 126641. [Google Scholar] [CrossRef]
- Valdés, H.; Padilla, E.E.; Zaror, C.A. Influence of Chemical Surface Characteristics of Natural Zeolite on Catalytic Ozone Abatement. Ozone Sci. Eng. 2011, 33, 279–284. [Google Scholar] [CrossRef]
- Lu, J.-L.; Wang, S.; Zhao, K.; Wang, T.; Ni, C.-J.; Wang, M.-Z.; Wang, S.-D. Study on catalytic performance of supported transition metal oxide catalyst for ozone decomposition. J. Fuel Chem. 2021, 49, 1014–1022. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Li, W.; Gibbs, G.V.; Oyama, S.T. Mechanism of Ozone Decomposition on a Manganese Oxide Catalyst. 1. In Situ Raman Spectroscopy and Ab Initio Molecular Orbital Calculations. J. Am. Chem. Soc. 1998, 120, 9041–9046. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, P.; Zhang, B.; Li, J.; Wang, M. Facile Synthesis of Activated Carbon-supported Porous Manganese Oxide via in situ Reduction of Permanganate for Ozone Decomposition. Ozone Sci. Eng. 2013, 35, 308–315. [Google Scholar] [CrossRef]
- Lian, Z.; Ma, J.; He, H. Decomposition of high-level ozone under high humidity over Mn–Fe catalyst: The influence of iron precursors. Catal. Commun. 2015, 59, 156–160. [Google Scholar] [CrossRef]
- Tidahy, H.L.; Siffert, S.; Lamonier, J.-F.; Cousin, R.; Zhilinskaya, E.A.; Aboukaïs, A.; Su, B.-L.; Frère, M.; Giraudon, J.-M.; Leclercq, G. Influence of the exchanged cation in Pd/BEA and Pd/FAU zeolites for catalytic oxidation of VOCs. Appl. Catal. B Environ. 2007, 70, 377–383. [Google Scholar] [CrossRef]
- Mendez, V.; Caps, V.; Daniele, S. Design of hybrid titania nanocrystallites as supports for gold catalysts. Chem. Commun. 2009, 21, 3116–3118. [Google Scholar] [CrossRef] [PubMed]
- Mehri, A.; Kochkar, H.; Daniele, S.; Mendez, V.; Ghorbel, A.; Berhault, G. One-pot deposition of palladium on hybrid TiO2 nanoparticles and catalytic applications in hydrogenation. J. Colloid Interface Sci. 2012, 369, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Klimov, O.V.; Pashigreva, A.V.; Fedotov, M.A.; Kochubey, D.I.; Chesalov, Y.A.; Bukhtiyarova, G.A.; Noskov, A.S. Co–Mo catalysts for ultra-deep HDS of diesel fuels prepared via synthesis of bimetallic surface compounds. J. Mol. Catal. A 2010, 322, 80–89. [Google Scholar] [CrossRef]
- Comparelli, R.; Fanizza, E.; Curri, M.L.; Cozzoli, P.D.; Mascolo, G.; Passino, R.; Agostiano, A. Photocatalytic degradation of azo dyes by organic-capped anatase TiO2 nanocrystals immobilized onto substrates. Appl. Catal. B Environ. 2005, 55, 81–91. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam reforming of methanol over Pd/ZnO: Effect of the formation of PdZn alloys upon the Reaction. Appl. Catal. A Gen. 1995, 125, 146. [Google Scholar] [CrossRef]
- Wu, Z.; Sheng, Z.; Liu, Y.; Wang, H.; Tang, N.; Wang, J. Characterization and activity of Pd-modified TiO2 catalysts for photocatalytic oxidation of NO in gas phase. J. Hazard. Mat. 2009, 164, 542–548. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Zhou, H.; Zhu, Y.; Sutter, E.; Ji, Y.; Rafailovich, M.H.; Sokolov, J.C. Seedless and Templateless Synthesis of Rectangular Palladium Nanoparticles. Chem. Mater. 2007, 19, 2065–2070. [Google Scholar] [CrossRef]
- Kameya, T.; Urano, K. Catalytic Decomposition of Ozone Gas by a Pd Impregnated MnO2 Catalyst. J. Environ. Eng. 2002, 128, 286–292. [Google Scholar] [CrossRef]
- Wu, M.C.; Kelly, N.A. Clean-air catalyst system for on-road applications: II. Mechanistic studies of pollutant removal. Appl. Catal. B Environ. 1998, 18, 93–104. [Google Scholar] [CrossRef]
- Rakitskaya, T.L.; Ennan, A.A.; Granatyuk, I.V.; Bandurko, A.Y.; Balavoine, G.G.; Geletii, Y.; Paina, V. Kinetics and mechanism of low-temperature ozone decomposition by Co-ions adsorbed on silica. Catal. Today 1999, 53, 715–723. [Google Scholar] [CrossRef]
- Gopi, T.; Swetha, G.; Chandra Shekar, S.; Ramakrishna, C.; Saini, B.; Krishna, R.; Rao, P.V.L. Catalytic decomposition of ozone on nanostructured potassium and proton containing δ-MnO2 catalysts. Catal. Comm. 2017, 92, 51–55. [Google Scholar] [CrossRef]
- Spasova, I.; Nikilov, P.; Mehandjiev, D. Ozone decomposition over alumina-supported copper, manganese and copper-manganese catalysts. Ozone Sci. Eng. 2007, 29, 41–45. [Google Scholar] [CrossRef]
- Pei, J.; Lu, Y.; Yin, X. Catalytic Decomposition of Ozone by CuO/MnO2-Performance, Kinetics and Application Analysis. Proc. Eng. 2015, 121, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Subrahmanyam, C.; Bulushev, D.A.; Kiwi-Minsker, L. Dynamic behavior of activated carbon catalysts during ozone decomposition at room temperature. Appl. Catal. B Environ. 2005, 61, 98–106. [Google Scholar] [CrossRef]
- Mehandjiev, D.; Naydenov, A.; Ivanov, G. Ozone decomposition, benzene and CO oxidation over NiMnO3-ilmenite and NiMn2O4-spinel catalysts. Appl. Catal. A Gen. 2001, 206, 13–18. [Google Scholar] [CrossRef]
- Shen, T.; Su, W.; Yang, Q.; Ni, J.; Tong, S. Synergetic mechanism for basic and acid sites of MgMxOy (M = Fe, Mn) double oxides in catalytic ozonation of p-hydroxybenzoic acid and acetic acid. Appl. Catal. B Environ. 2020, 279, 119346. [Google Scholar] [CrossRef]
- Mehandjiev, D.; Naydenov, A. Ozone decomposition on α-Fe2O3 catalyst. Ozone Sci. Eng. 1992, 14, 277–282. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, J.; Jiang, W.; Zhang, Z.; Wang, J.; Zhu, Y.; Zhang, Q. Surface oxygen vacancy induced-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, L.; Shang, H.; Chen, Y. Effect of preparation method on the performance of Pd-MnOx/γ-Al2O3 monolithic catalysts for ground-level O3 decomposition. Chin. J. Catal. 2014, 35, 1883–1991. [Google Scholar] [CrossRef]









| Catalyst | Pd a (wt%) | Pd 3d5/2 (XPS) | Pd (at.%) | Pd/Ti (XPS) | O (%) | N b (%) | C b (%) |
|---|---|---|---|---|---|---|---|
| Pd°/PdOx (%) | |||||||
| Pd/TiO2_20 | 0.40 | 75/25 | 0.05 | 0.0033 | 52 | 0.04 | 23.83 (4.80) |
| Pd/TiO2_50 | 0.36 | 71/29 | 0.08 | 0.0044 | 57 | 0.02 | 19.60 (3.04) |
| Pd/TiO2_100 | 0.32 | 70/30 | 0.10 | 0.0062 | 58 | 0.01 | 17.05 (1.80) |
| Pd/TiO2_citrate free | 0.39 | nd/nd | nd | nd | nd | nd |
| Catalyst | SBET (m2 g−1) | Dp (nm) | Vp (cm3 g−1) | CBET a | dXRD (nm) | d (nm) b |
|---|---|---|---|---|---|---|
| Pd/TiO2_20 | 279 (335) | 25 (25) | 0.18 (0.13) | 116 (116) | 5.0 (6.0) | 6 |
| Pd/TiO2_50 | 268 (303) | 35 (33) | 0.20 (0.19) | 72 (71) | 6.0 (6.5) | 3 |
| Pd/TiO2_100 | 223 (263) | 70 (75) | 0.31 (0.29) | 70 (70) | 6.5 (7.0) | 4 |
| Pd/TiO2_citrate free | 232 (212) | 65 (65) | 0.49 (0.40) | 103 (109) | 9.0 (8.5) | 12 |
| TOF (min−1) | ||
|---|---|---|
| Catalyst | Dry | Humid |
| Pd/TiO2_20 | 235 | 235 |
| Pd/TiO2_50 | 306 | 304 |
| Pd/TiO2_100 | 349 | 349 |
| Pd/TiO2_citrate free | 142 | 130 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touati, H.; Mehri, A.; Karouia, F.; Richard, F.; Batiot-Dupeyrat, C.; Daniele, S.; Clacens, J.-M. Low-Temperature O3 Decomposition over Pd-TiO2 Hybrid Catalysts. Catalysts 2022, 12, 448. https://doi.org/10.3390/catal12040448
Touati H, Mehri A, Karouia F, Richard F, Batiot-Dupeyrat C, Daniele S, Clacens J-M. Low-Temperature O3 Decomposition over Pd-TiO2 Hybrid Catalysts. Catalysts. 2022; 12(4):448. https://doi.org/10.3390/catal12040448
Chicago/Turabian StyleTouati, Houcine, Afef Mehri, Fathi Karouia, Frédéric Richard, Catherine Batiot-Dupeyrat, Stéphane Daniele, and Jean-Marc Clacens. 2022. "Low-Temperature O3 Decomposition over Pd-TiO2 Hybrid Catalysts" Catalysts 12, no. 4: 448. https://doi.org/10.3390/catal12040448
APA StyleTouati, H., Mehri, A., Karouia, F., Richard, F., Batiot-Dupeyrat, C., Daniele, S., & Clacens, J. -M. (2022). Low-Temperature O3 Decomposition over Pd-TiO2 Hybrid Catalysts. Catalysts, 12(4), 448. https://doi.org/10.3390/catal12040448