Optimization of Liquid Hot Water Pretreatment and Fermentation for Ethanol Production from Sugarcane Bagasse Using Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Sugarcane Bagasse Substrates
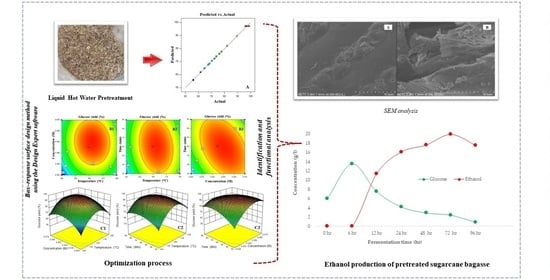
2.2. Optimization of Reaction Temperature, Residual Time, and Acid Concentration for Glucose Yield
2.3. Optimization of Glucose Yield after Liquid Hot Water Pretreatment of Sugarcane Bagasse
2.4. SEM, XRD, BET Surface, and FTIR Characterization of Native Sugarcane Bagasse and Solid Residue after LHW Pretreatment
2.5. Simultaneous Saccharification and Fermentation (SSF)
3. Materials and Methods
3.1. Materials
3.2. Liquid Hot Water Pretreatment of Sugarcane Bagasse
3.3. Experimental Design and Optimization of Glucose Yield Using Box–Behnken Response Surface Design
3.4. Enzymatic Hydrolysis
3.5. Analysis of Aqueous Phase
3.6. Characterization of Native Sugarcane Bagasse and Remaining Solid Residue
3.6.1. Scanning Electron Microscopy Analysis
3.6.2. X-ray Diffraction Analysis
3.6.3. BET Surface Area Measurement
3.6.4. Fourier-Transform Infrared Spectroscopy Analysis
3.7. Simultaneous Saccharification and Fermentation Process for Ethanol Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mesa, L.; González, E.; Cara, C.; Ruiz, E.; Castro, E.; Mussatto, S.I. An approach to optimization of enzymatic hydrolysis from sugarcane bagasse based on organosolv pretreatment. J. Chem. Technol. Biotechnol. 2010, 85, 1092–1098. [Google Scholar] [CrossRef]
- Busic, A.; Mardetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivancic Santek, M.; Santek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Lobo, M.G.; Dorta, E. Utilization and Management of Horticultural Waste. In Postharvest Technology of Perishable Horticultural Commodities; Woodhead Publishing: Sawston, UK, 2019; pp. 639–666. [Google Scholar]
- Sindhu, R.; Binod, P.; Pandey, A.; Ankaram, S.; Duan, Y.; Awasthi, M.K. Chapter 5—Biofuel Production from Biomass: Toward Sustainable Development. In Current Developments in Biotechnology and Bioengineering; Kumar, S., Kumar, R., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–92. [Google Scholar]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Benaimeche, O.; Seghir, N.T.; Sadowski, Ł.; Mellas, M. The Utilization of Vegetable Fibers in Cementitious Materials. In Encyclopedia of Renewable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 649–662. [Google Scholar]
- French, A.D. Combining computational chemistry and crystallography for a better understanding of the structure of cellulose. Adv. Carbohydr. Chem. Biochem. 2012, 67, 19–93. [Google Scholar] [PubMed]
- Kim, I.; Han, J.-I. Optimization of alkaline pretreatment conditions for enhancing glucose yield of rice straw by response surface methodology. Biomass Bioenergy 2012, 46, 210–217. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Lei, F.; Jiang, J. Co-production bioethanol and xylooligosaccharides from sugarcane bagasse via autohydrolysis pretreatment. Renew. Energy 2020, 162, 2297–2305. [Google Scholar] [CrossRef]
- Milessi, T.S.; Corradini, F.A.S.; Marçal, J.V.M.; Baldez, T.O.; Kopp, W.; Giordano, R.C.; Giordano, R.L.C. Xylooligosaccharides production chain in sugarcane biorefineries: From the selection of pretreatment conditions to the evaluation of nutritional properties. Ind. Crops Prod. 2021, 172, 114056. [Google Scholar] [CrossRef]
- Silva, G.; Giordano, R.L.C.; Cruz, A.; Ramachandriya, K.D.; Banat, I.; Wilkins, M. Ethanol production from sugarcane bagasse using SSF process and thermotolerant yeast. Trans. ASABE 2015, 58, 193–200. [Google Scholar]
- Yamada, R.; Taniguchi, N.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Direct ethanol production from cellulosic materials using a diploid strain of Saccharomyces cerevisiae with optimized cellulase expression. Biotechnol. Biofuels 2011, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Lü, X. Chapter 8—Overcome saccharification barrier: Advances in hydrolysis technology. In Advances in 2nd Generation of Bioethanol Production; Lü, X., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 137–159. [Google Scholar]
- Lima, M.A.; Gomez, L.D.; Steele-King, C.G.; Simister, R.; Bernardinelli, O.D.; Carvalho, M.A.; Rezende, C.A.; Labate, C.A.; Deazevedo, E.R.; McQueen-Mason, S.J. Evaluating the composition and processing potential of novel sources of Brazilian biomass for sustainable biorenewables production. Biotechnol. Biofuels 2014, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Liu, J.; Zheng, P.; Li, M.; Zhou, Y.; Huang, L.; Shuai, L. Promoting enzymatic hydrolysis of lignocellulosic biomass by inexpensive soy protein. Biotechnol. Biofuels 2019, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.-B.; Wang, L.; Liu, D.-H. Peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis: A continued work. J. Chem. Technol. Biotechnol. 2008, 83, 950–956. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Popescu, C.M.; Popescu, M.C.; Vasile, C. Structural analysis of photodegraded lime wood by means of FT-IR and 2D IR correlation spectroscopy. Int. J. Biol. Macromol. 2011, 48, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Piqueras, S.; Fuchtner, S.; Rocha de Oliveira, R.; Gomez-Sanchez, A.; Jelavic, S.; Keplinger, T.; Thygesen, L.G. Understanding the Formation of Heartwood in Larch Using Synchrotron Infrared Imaging Combined with Multivariate Analysis and Atomic Force Microscope Infrared Spectroscopy. Front. Plant Sci. 2019, 10, 1701. [Google Scholar] [CrossRef]
- Zhou, G.; Taylor, G.; Polle, A. FTIR-ATR-based prediction and modelling of lignin and energy contents reveals independent intra-specific variation of these traits in bioenergy poplars. Plant Methods 2011, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Ishola, M.M.; Millati, R.; Syamsiah, S.; Cahyanto, M.N.; Niklasson, C.; Taherzadeh, M.J. Structural changes of oil palm empty fruit bunch (OPEFB) after fungal and phosphoric acid pretreatment. Molecules 2012, 17, 14995–15002. [Google Scholar]
- Fan, M.; Zhao, C.; Huang, X.; Zhang, H.; Xie, J. Enhanced digestibility and fermentability of sugarcane bagasse in biofuel production by surfactant-assisted dilute acid pretreatment. Ind. Crops Prod. 2021, 172, 114006. [Google Scholar] [CrossRef]
- Wang, W.; Zhuang, X.; Yuan, Z.; Yu, Q.; Xu, J.; Qi, W.; Wang, Q. Highly efficient conversion of sugarcane bagasse pretreated with liquid hot water into ethanol at high solid loading. Int. J. Green Energy 2016, 13, 298–304. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory NREL/TP-510-42623: Denver, CO, USA, 2008. [Google Scholar]
- Weerasai, K.; Suriyachai, N.; Poonsrisawat, A.; Arnthong, J.; Unrean, P.; Laosiripojana, N.; Champreda, V. Sequential Acid and Alkaline Pretreatment of Rice Straw for Bioethanol Fermentation. BioResources 2014, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Thailand Institute of Scientific and Technological Research (TISTR Culture Collection). Available online: https://www.tistr.or.th/tistr_culture/list_en.php?type=y&key=S (accessed on 10 February 2020).




| Factors | Responses (%) | ||||||
|---|---|---|---|---|---|---|---|
| T (°C) | Acid Concentration (M) | Time (min) | Cellulose (%) a | Hemicellulose (%) a | Lignin (%) a | Remaining Solid Residual (%) a | Glucose Yield (%) |
| 140 | 0.025 | 60 | 26.32 | 2.11 | 29.30 | 72.47 | 56.07 |
| 180 | 0.025 | 60 | 26.34 | 0.63 | 22.60 | 69.87 | 67.54 |
| 140 | 0.075 | 60 | 25.54 | 0.87 | 25.76 | 76.89 | 68.43 |
| 180 | 0.075 | 60 | 23.62 | 0.27 | 22.50 | 56.44 | 73.69 |
| 140 | 0.050 | 30 | 28.89 | 2.52 | 28.12 | 65.46 | 61.68 |
| 180 | 0.050 | 30 | 26.32 | 1.21 | 27.65 | 66.21 | 77.78 |
| 140 | 0.050 | 90 | 26.70 | 1.90 | 28.78 | 79.86 | 70.32 |
| 180 | 0.050 | 90 | 27.61 | 0.23 | 24.56 | 50.45 | 72.35 |
| 160 | 0.025 | 30 | 28.43 | 2.04 | 24.00 | 79.44 | 64.67 |
| 160 | 0.075 | 30 | 25.88 | 1.50 | 23.18 | 69.51 | 89.58 |
| 160 | 0.025 | 90 | 27.83 | 0.89 | 25.13 | 69.35 | 81.97 |
| 160 | 0.075 | 90 | 26.39 | 0.09 | 23.35 | 70.57 | 76.09 |
| 160 | 0.050 | 60 | 29.40 | 0.59 | 23.50 | 67.37 | 96.86 |
| 160 | 0.050 | 60 | 29.43 | 0.61 | 23.49 | 67.43 | 96.12 |
| 160 | 0.050 | 60 | 29.38 | 0.60 | 23.54 | 67.33 | 97.13 |
| 160 | 0.050 | 60 | 29.42 | 0.64 | 23.51 | 67.29 | 96.72 |
| 160 | 0.050 | 60 | 29.46 | 0.66 | 23.73 | 67.44 | 98.85 |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value Prob > F | Comments |
|---|---|---|---|---|---|---|
| Model | ||||||
| A-Temperature | 151.99 | 1 | 151.99 | 233.95 | <0.0001 | Significance |
| B-Concentration | 176.15 | 1 | 176.15 | 271.14 | <0.0001 | Significance |
| C-Time | 6.15 | 1 | 6.15 | 9.47 | 0.0179 a | |
| AB | 9.64 | 1 | 9.64 | 14.84 | 0.0063 a | |
| AC | 49.56 | 1 | 49.56 | 76.29 | <0.0001 | Significance |
| BC | 237.01 | 1 | 237.01 | 364.81 | <0.0001 | Significance |
| A*2 | 1539.83 | 1 | 1539.83 | 2370.18 | <0.0001 | Significance |
| B*2 | 564.62 | 1 | 564.62 | 869.09 | <0.0001 | Significance |
| C*2 | 235.48 | 1 | 235.48 | 362.47 | <0.0001 | Significance |
| Order | Surface Area (m2/g) | Degree of Crystallinity (%) |
|---|---|---|
| Native sugarcane bagasse | 2.1 | 49.6 |
| Solid residuals after LHW pretreatment | 10.5 | 66.8 |
| Order | Frequency, (cm−1) | Functional Group |
|---|---|---|
| 1 | 3449–3431 | O–H stretching in phenolic compound |
| 2 | 2915–2895 | C–H stretching vibrations in methyl group |
| 3 | 1609–1602 | C–O stretching in lignin structure |
| 4 | 1375–1370 | C–H deformation in cellulose and hemicellulose |
| 5 | 1429–1428 | CH-2 stretching vibrations in cellulose |
| 6 | 1221–1220 | C–O stretch of syringyl rings in lignin structure |
| 7 | 1164–1162 | C–O–C vibration of cellulose and hemicellulose |
| 8 | 1130–1128 | automatic structure in lignin structure |
| 9 | 1059–1043 | C–O stretch vibrations in cellulose |
| 10 | ~895 | C–H–O stretching vibrations of β-(1-4)-glycosidic linkage |
| 11 | ~751 | CH-2 bands |
| Run | T (°C) | Acid Concentration (M) | Time (min) |
|---|---|---|---|
| 1 | −1 | −1 | 0 |
| 2 | 1 | −1 | 0 |
| 3 | −1 | 1 | 0 |
| 4 | 1 | 1 | 0 |
| 5 | −1 | 0 | −1 |
| 6 | 1 | 0 | −1 |
| 7 | −1 | 0 | 1 |
| 8 | 1 | 0 | 1 |
| 9 | 0 | −1 | −1 |
| 10 | 0 | 1 | −1 |
| 11 | 0 | −1 | 1 |
| 12 | 0 | 1 | 1 |
| 13 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 |
| 17 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khongchamnan, P.; Suriyachai, N.; Kreetachat, T.; Laosiripojana, N.; Weerasai, K.; Champreda, V.; Suwannahong, K.; Sakulthaew, C.; Chokejaroenrat, C.; Imman, S. Optimization of Liquid Hot Water Pretreatment and Fermentation for Ethanol Production from Sugarcane Bagasse Using Saccharomyces cerevisiae. Catalysts 2022, 12, 463. https://doi.org/10.3390/catal12050463
Khongchamnan P, Suriyachai N, Kreetachat T, Laosiripojana N, Weerasai K, Champreda V, Suwannahong K, Sakulthaew C, Chokejaroenrat C, Imman S. Optimization of Liquid Hot Water Pretreatment and Fermentation for Ethanol Production from Sugarcane Bagasse Using Saccharomyces cerevisiae. Catalysts. 2022; 12(5):463. https://doi.org/10.3390/catal12050463
Chicago/Turabian StyleKhongchamnan, Punjarat, Nopparat Suriyachai, Torpong Kreetachat, Navadol Laosiripojana, Khatiya Weerasai, Verawat Champreda, Kowit Suwannahong, Chainarong Sakulthaew, Chanat Chokejaroenrat, and Saksit Imman. 2022. "Optimization of Liquid Hot Water Pretreatment and Fermentation for Ethanol Production from Sugarcane Bagasse Using Saccharomyces cerevisiae" Catalysts 12, no. 5: 463. https://doi.org/10.3390/catal12050463
APA StyleKhongchamnan, P., Suriyachai, N., Kreetachat, T., Laosiripojana, N., Weerasai, K., Champreda, V., Suwannahong, K., Sakulthaew, C., Chokejaroenrat, C., & Imman, S. (2022). Optimization of Liquid Hot Water Pretreatment and Fermentation for Ethanol Production from Sugarcane Bagasse Using Saccharomyces cerevisiae. Catalysts, 12(5), 463. https://doi.org/10.3390/catal12050463