Hollow Nanospheres Organized by Ultra-Small CuFe2O4/C Subunits with Efficient Photo-Fenton-like Performance for Antibiotic Degradation and Cr(VI) Reduction
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
3.1. Structure and Composition Characterization
3.2. Evaluation of Photocatalytic Performance
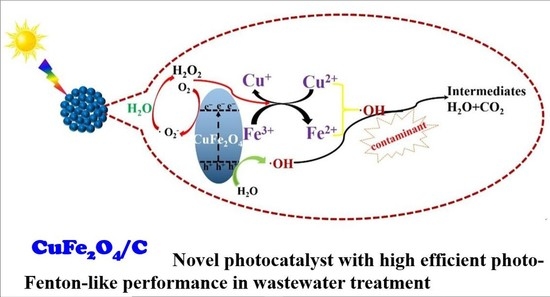
3.3. The Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lei, C.; Pi, M.; Jiang, C.; Cheng, B.; Yu, J. Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface Sci. 2017, 490, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jing, L.; Teng, Y.; Wang, J. Multimedia fate modeling and risk assessment of antibiotics in a water-scarce megacity. J. Hazard. Mater. 2018, 348, 75–83. [Google Scholar] [CrossRef]
- Choe, J.Y.; Byun, J.Y.; Kim, S.H. Fe3S4/Fe7S8-promoted degradation of phenol via heterogeneous, catalytic H2O2 scission mediated by S-modified surface Fe2+ species. Appl. Catal. B Environ. 2018, 233, 272–280. [Google Scholar] [CrossRef]
- Azhar, M.R.; Abid, H.R.; Sun, H.; Periasamy, V.; Tadé, M.O.; Wang, S. Excellent performance of copper based metal organic framework in adsorptive removal of toxic sulfonamide antibiotics from wastewater. J. Colloid Interface Sci. 2016, 478, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Talaiekhozani, A.; Talaei, M.R.; Rezania, S. An Overview on production and application of ferrate (VI) for chemical oxidation, coagulation and disinfection of water and wastewate. J. Environ. Chem. Eng. 2017, 5, 1828–1842. [Google Scholar] [CrossRef]
- Dong, J.; Feng, W.; Wang, L.; Li, M.; Chen, Z.; Xu, X. Cu/base co-catalyzed [3+3] cycloaddition for the synthesis of highly functionalized 4-fluoropyridines. Chem. Commun. 2021, 57, 12635–12638. [Google Scholar] [CrossRef]
- Seng, R.; Tan, L.; Lee, W.P.C.; Ong, W.J.; Chai, S. Nitrogen-doped carbon quantum dots-decorated 2D graphitic carbon nitride as a promising photocatalyst for environmental remediation: A study on the importance of hybridization approach. J. Environ. Manag. 2020, 255, 109936. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, F.; Chen, Z.; Shi, H. NixCo1-xS as an effective noble metal free cocatalyst for enhanced photocatalytic activity of g-C3N4. J. Mater. Sci. Technol. 2020, 56, 227–235. [Google Scholar] [CrossRef]
- Chen, Z.; Bin, Y.; Tian, F.; Zhu, B. NiS and graphene as dual cocatalysts for the enhanced photocatalytic H2 production activity of g-C3N4. Appl. Surf. Sci. 2018, 469, 657–665. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon dioxide removal through Physical Adsorption using Carbonaceous and non-Carbonaceous Adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Murray, A.; Örmeci, B. Use of polymeric sub-micron ion-exchange resins for removal of lead, copper, zinc, and nickel from natural waters. J. Environ. Sci. 2019, 75, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhao, T.; Wang, J.; Wang, Y.; Chen, Z.; Liu, B.; Ji, H.; Wang, W.; Zhang, G.; Li, Y. Fabrication of g-C3N4/PW12/TiO2 composite with significantly enhanced photocatalytic performance under visible light. J. Alloys Compd. 2021, 860, 157924. [Google Scholar] [CrossRef]
- Wang, K.; Sheng, Y.; Cao, H.; Yan, K.; Zhang, Y. Impact of applied current on sulfate-rich wastewater treatment and microbial biodiversity in the cathode chamber of microbial electrolysis cell (MEC) reactor. Chem. Eng. J. 2017, 307, 150–158. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Tian, Z.; Yang, M.; Zhang, Y. Characteristics of ARG-carrying plasmidome in the cultivable microbial community from wastewater treatment system under high oxytetracycline concentration. Appl. Microbiol. Biotechnol. 2018, 102, 1847–1858. [Google Scholar] [CrossRef]
- Li, J.; Luo, C.; Song, M.; Dai, Q.; Jiang, L.; Zhang, D.; Zhang, G. Biodegradation of phenanthrene in polycyclic aromatic hydrocarbon-contaminated wastewater revealed by coupling cultivation-dependent and -independent approaches. Environ. Sci. Technol. 2017, 51, 3391. [Google Scholar] [CrossRef]
- Peng, D.; Wang, Y.; Shi, H.; Jiang, W.; Jin, T.; Jin, H.; Chen, Z. Fabrication of novel Cu2WS4/NiTiO3 heterostructures for efficient visible-light photocatalytic hydrogen evolution and pollutant degradation. J. Colloid Interface Sci. 2022, 613, 194–206. [Google Scholar] [CrossRef]
- Mu, D.; Chen, Z.; Chen, F.; Shi, H. Construction of flower-like MoS2/Fe3O4/rGO composite with enhanced photo-Fenton like catalyst performance. RSC Adv. 2018, 8, 36625–36631. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, C.; Zeng, G.; Tan, X.; Wang, H.; Huang, D.; Yang, K.; Wei, J.; Ma, C.; Nie, K. Design and engineering Layered Double Hydroxide based catalysts for water depollution by advanced oxidation processes: A review. J. Mater. Chem. A 2020, 8, 4141–4173. [Google Scholar] [CrossRef]
- Chuang, Y.; Chen, S.; Chinn, C.; Mitch, W. Comparing the UV/monochloramine and UV/free chlorine Advanced Oxidation Processes (AOPs) to the UV/hydrogen peroxide AOP Under Scenarios Relevant to Potable Reuse. Environ. Sci. Technol. 2017, 51, 13859–13868. [Google Scholar] [CrossRef]
- Li, X.; Zhou, M.; Pan, Y.; Xu, L.; Tang, Z. Highly efficient advanced oxidation processes (AOPs) based on pre-magnetization Fe0 for wastewater treatment. Sep. Purif. Technol. 2017, 178, 49–55. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, Y.; Wang, Y.; Sun, S.; Wu, W.; Wu, Z. Nanostructured semiconductor supported iron catalysts for heterogeneous photo-fenton oxidation: A review. J. Mater. Chem. A 2020, 8, 15513–15546. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Bastidas-G, K.; Sierra, C.A.; Ramirez, H.R.Z. Heterogeneous Fenton oxidation of Orange II using iron nanoparticles supported on natural and functionalized fique fiber. J. Environ. Chem. Eng. 2018, 6, 4178–4188. [Google Scholar] [CrossRef]
- Qin, Y.; Faheem, A.; Jia, G.; Hu, Y. Self-assembled Fe3+@spores as a sustainable heterogeneous Fenton catalyst for arsenite removal. J. Environ. Chem. Eng. 2020, 8, 104485. [Google Scholar] [CrossRef]
- Chen, F.; Xie, S.; Huang, X.; Qiu, X. Ionothermal synthesis of Fe3O4 magnetic nanoparticles as efficient heterogeneous Fenton-like catalysts for degradation of organic pollutants with H2O2. J. Hazard. Mater. 2017, 322, 152–162. [Google Scholar] [CrossRef]
- Filice, S.; Corrado, B.; Libertino, S.; Gradon, L.; Iannazzo, D.; Scalese, S. Photo-Fenton degradation of methyl orange with dunino halloysite as a source of iron heterogeneous. Catalysts 2022, 12, 257. [Google Scholar] [CrossRef]
- Qian, X.; Ren, M.; Zhu, Y.; Yue, D.; Han, Y.; Jia, J.; Zhao, Y. Visible Light Assisted Heterogeneous Fenton-like Degradation of Organic Pollutant via α-FeOOH/Mesoporous Carbon Composites. Environ. Sci. Technol. 2017, 51, 3993–4000. [Google Scholar] [CrossRef]
- Jin, H.; Tian, X.; Nie, Y.; Zhou, Z.; Yang, C.; Li, Y.; Lu, L. Oxygen vacancy promoted heterogeneous fentonlike degradation of ofloxacin at pH 3.2-9.0 by Cu substituted magnetic Fe3O4@FeOOH nanocomposite. Environ. Sci. Technol. 2017, 51, 12699–12706. [Google Scholar] [CrossRef]
- Khandarkhaeva, M.; Batoeva, A.; Aseev, D.; Sizykh, M.; Tsydenova, O. Oxidation of atrazine in aqueous media by solar-enhanced Fenton-like process involving persulfate and ferrous ion. Ecotoxicol. Environ. Saf. 2017, 137, 35–41. [Google Scholar] [CrossRef]
- Fei, B.; Deng, N.; Wang, J.; Liu, Q.; Long, J.; Li, Y.; Mei, X. A heteropoly blue as environmental friendly material: An excellent heterogeneous Fenton-like catalyst and flocculent. J. Hazard. Mater. 2017, 340, 326–335. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xie, Y.; Zeng, Y.; Li, P.; Xie, T.; Wang, Y. Ultrasonic-enhanced Fenton-like degradation of bisphenol A using a bio-synthesized schwertmannite catalyst. J. Hazard. Mater. 2018, 344, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Koltsakidou, A.; Antonopoulou, M.; Sykiotou, M.; Εvgenidou, Ε.; Konstantinou, I.; Lambropoulou, D.A. Photo-Fenton and Fenton-like processes for the treatmentof the antineoplastic drug 5-fluorouracil under simulated solar radiation. Environ. Sci. Pollut. Res. 2017, 24, 4791–4800. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Quan, X.; Wang, J.; Gao, C.; Chen, S.; Yu, H.; Zhang, Y. Enhanced heterogeneous Fenton-like activity by Cu-doped BiFeO3 perovskite for degradation of organic pollutants. Front. Environ. Sci. Eng. 2018, 12, 103–112. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Zhang, J.; Zhang, Z.; Tang, Y. Degradation of hydroxypropyl guar gum at wide pH range by a heterogeneous Fenton-like process using bentonite-supported Cu(0). Prog. Water Technol. 2020, 82, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Rishi, V.; Gupta, T. Synthesis of MFe2O4 (M: Cu, Mn, Co, Ni) magnetic nanoparticles and their efficient catalytic role in nitrophenol reduction. Mater. Res. Innovations 2021, 25, 393–398. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Bahadur, D.; Aslam, M. Surface controlled synthesis of MFe2O4 (M = Mn, Fe, Co, Ni and Zn) nanoparticles and their magnetic characteristics. Crystengcomm 2012, 15, 524–532. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Chen, F.; Shi, H. Recyclable magnetic NiFe2O4/C yolk–shell nanospheres with excellent visible-light-Fenton degradation performance of tetracycline hydrochloride. Dalton Trans. 2019, 48, 3038–3044. [Google Scholar]
- Dippong, T.; Levei, E.; Cadar, O. Recent Advances in Synthesis and Applications of MFe2O4 (M = Co, Cu, Mn, Ni, Zn) Nanoparticles. Nanomaterials 2021, 11, 1560. [Google Scholar] [CrossRef]
- Wang, L.; Bock, D.C.; Li, J.; Stach, E.A.; Marschilok, A.; Takeuchi, K.J.; Takeuchi, E.S. Synthesis and Characterization of CuFe2O4 Nano/sub-micron Wires-Carbon Nanotube Composites as Binder-free Anodes for Li-ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 8770–8785. [Google Scholar] [CrossRef]
- Park, S.; Baek, J.H.; Zhang, L.; Lee, J.M.; Stone, K.H.; Cho, I.S.; Guo, J.H.; Jung, H.S.; Zheng, X.L. Rapid Flame-annealed CuFe2O4 as Efficient Photocathode for Photoelectrochemical Hydrogen Production. ACS Sustain. Chem. Eng. 2019, 7, 5867–5874. [Google Scholar] [CrossRef]
- Qi, F.; Chu, W.; Xu, B. Comparison of phenacetin degradation in aqueous solutions by catalytic ozonation with CuFe2O4 and its precursor: Surface properties, intermediates and reaction mechanisms. Chem. Eng. J. 2016, 284, 28–36. [Google Scholar] [CrossRef]
- Bavandpour, R.; Karimi-Maleh, H.; Asif, M.; Atar, V.K.G.N.; Abbasghorbani, M. Liquid phase determination of adrenaline uses a voltammetric sensor employing CuFe2O4 nanoparticles and room temperature ionic liquids. J. Mol. Liq. 2016, 213, 369–373. [Google Scholar] [CrossRef]
- Marinca, T.F.; Chicinas, I.; Isnard, O. Synthesis, structural and magnetic characterization of nanocrystalline CuFe2O4 as obtained by a combined method reactive milling, heat treatment and ball milling. Ceram. Int. 2012, 38, 1951–1957. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Kuntalue, B.; Thongtem, S.; Thongtem, T. Synthesis of cubic CuFe2O4 nanoparticles by microwave-hydrothermal method and their magnetic properties. Mater. Lett. 2016, 167, 65–68. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, D.; Wang, C.; Diao, G. Facile fabrication of hierarchically porous CuFe2O4 nanospheres with enhanced capacitance property. ACS Appl. Mater. Interfaces 2013, 5, 6030–6037. [Google Scholar] [CrossRef]
- Jiang, J.; Ai, L. Microemulsion-mediated in-situ synthesis and magnetic characterization of polyaniline/Zn0.5Cu0.5Fe2O4 nanocomposite. Appl. Phys. A 2008, 92, 341–344. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.H.; Huang, K.L.; Chen, H. Photocatalytic Inactivation of the Bacteria Escherichia Coli by CuCr2O4/TiO2 Composite Photocatalysts under Simulated Solar Light Irradiation. Adv. Mater. Res. 2012, 343–344, 838–843. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Bharathi, G.; Pazhanivel, T.; Dhanalakshmi, M. Improved photocatalytic performance of magnetically recoverable Bi2Te3/CdS/CuFe2O4 nanocomposite for MB dye under visible light exposure. Solid State Sci. 2021, 115, 106584. [Google Scholar] [CrossRef]
- Li, X.; Lu, D.; Shao, C.; Lu, G.; Li, X.; Liu, Y. Hollow CuFe2O4/-Fe2O3 composite with ultrathin porous shell for acetone detection at ppb levels. Sens. Actuators B 2018, 258, 436–446. [Google Scholar] [CrossRef]
- Kucera, M.; Brom, P. Magneto-optical properties of nano-crystalline cubic and tetragonal copper ferrite thin films. J. Appl. Phys. 2015, 117, 738. [Google Scholar] [CrossRef]
- Samson, V.A.F.; Bernadsha, S.B.; Mahendiran, M.; Lawrence, K.L.; Madhavan, J.; Raj, M.V.A.; Prathap, S. Impact of calcination temperature on structural, optical, and magnetic properties of spinel CuFe2O4 for enhancing photocatalytic activity. J. Mater. Sci. 2020, 31, 6574–6585. [Google Scholar] [CrossRef]
- Hariganesh, S.; Vadivel, S.; Paul, B.; Kumaravel, M.; Balasubramanian, N.; Rajendran, S.; Dhar, S.S. Metal organic framework derived magnetically recoverable CuFe2O4 porous cubes for efficient photocatalytic application. Inorg. Chem. Commun. 2021, 125, 108405. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, J.H.; Lee, S.H. Magneto-optical investigation of spinel ferrite CuFe2O4: Observation of Jahn–Teller effect in Cu2+ ion. J. Magn. Magn. Mater. 2004, 279, 173–177. [Google Scholar] [CrossRef]
- Selima, S.S.; Khairy, M.; Mousa, M.A. Comparative studies on the impact of synthesis methods on structural, optical, magnetic and catalytic properties of CuFe2O4. Ceram. Int. 2019, 45, 6535–6540. [Google Scholar] [CrossRef]
- Nawle, A.C.; Humbe, A.V.; Babrekar, M.K.; Deshmukh, S.S.; Jadhav, K.M. Deposition, characterization, magnetic and optical properties of Zn doped CuFe2O4 thin films. J. Alloys Compd. 2017, 695, 1573–1582. [Google Scholar] [CrossRef]
- Rani, B.J.; Saravanakumar, B.; Ravi, G.; Ganesh, V.; Ravichandran, S.; Yuvakkumar, R. Structural, optical and magnetic properties of CuFe2O4 nanoparticles. J. Mater. Sci. 2018, 29, 1975–1984. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, D.; Hou, C.; Liang, C.; Li, H. Facile one-pot synthesis of cellulose nanocrystal-supported hollow CuFe2O4 nanoparticles as efficient catalyst for 4-nitrophenol reduction. J. Nanopart. Res. 2018, 20, 161. [Google Scholar] [CrossRef]
- Guo, X.; Wang, K.; Li, D.; Qin, J. Heterogeneous Photo-Fenton Processes Using Graphite Carbon Coating Hollow CuFe2O4 Spheres for the Degradation of Methylene Blue. Appl. Surf. Sci. 2017, 420, 792–801. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, C.; Shih, K. Copper-promoted circumneutral activation of H2O2 by magnetic CuFe2O4 spinel nanoparticles: Mechanism, stoichiometric efficiency, and pathway of degrading sulfanilamide. Chemosphere 2016, 154, 573–582. [Google Scholar] [CrossRef]
- Yao, Y.; Lu, F.; Chen, H.; Wei, F.; Liu, X.; Lian, C.; Wang, S. Magnetic core–shell CuFe2O4@C3N4 hybrids for visible light photocatalysis of orange II. J. Hazard. Mater. 2015, 297, 224–233. [Google Scholar] [CrossRef]
- Bian, L.; Nie, J.; Jiang, X.; Song, M.; Dong, F.; Li, W.; Shang, L.; Deng, H.; He, H.; Xu, B.; et al. Selective removal of uranyl from aqueous solutions containing mix of toxic metal ions using core/shell MFe2O4-TiO2 nanoparticles of montmorillonite edge sites. ACS Sustain. Chem. Eng. 2018, 6, 16267–16278. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, C.; Bi, H.; Liu, Y.; Yan, Q. Magnetically separable CuFe2O4/AgBr composite photocatalysts: Preparation, characterization, photocatalytic activity and photocatalytic mechanism under visible light. Appl. Surf. Sci. 2017, 392, 701–707. [Google Scholar] [CrossRef]
- Shi, F.; Shan, H.; Li, D.; Yin, X.; Yu, J.; Ding, B. A general strategy to fabricate soft magnetic CuFe2O4@SiO2 nanofibrous membranes as efficient and recyclable Fenton-like catalysts. J. Colloid Interface Sci. 2019, 538, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, J.; Ge, M. Synthesis of magnetic Cu/CuFe2O4 nanocomposite as a highly efficient Fenton-like catalyst for methylene blue degradation. J. Mater. Sci. 2018, 53, 15081–15095. [Google Scholar] [CrossRef]
- Enneffati, M.; Rasheed, M.; Louati, B.; Guidara, K.; Barillé, R. Morphology, UV–visible and ellipsometric studies of sodium lithium orthovanadate. J. Opt. Quant. Electron. 2019, 51, 299. [Google Scholar] [CrossRef]
- Chutia, R.; Chetia, B. Ligand and additive free aerobic synthesis of diynes using Pd–CuFe2O4 magnetic nanoparticles as an efficient reusable catalyst. New J. Chem. 2020, 44, 18199–18207. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, W.; Jing, G.; Zhou, Z. Activation of sulfite autoxidation with CuFe2O4 prepared by MOF templated method for abatement of organic contaminants. Environ. Pollut. 2020, 260, 114038. [Google Scholar] [CrossRef]
- Das, S.; Patnaik, S.; Parida, K. Dynamic charge transfer through Fermi level equilibration in the p-CuFe2O4/n-NiAl LDH interface towards photocatalytic application. Catal. Sci. Technol. 2020, 10, 6285–6298. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Mathew, T.; Shi, J.; Sreekumar, K.; Rao, B.; Gopinath, C. Cu-Co Synergism in Cu1-xCoxFe2O4-Catalysis and XPS Aspects. J. Catal. 2002, 210, 405–417. [Google Scholar] [CrossRef]
- Wang, J.; Yu, B.; Wang, W.; Cai, X. Facile synthesis of carbon dots-coated CuFe2O4 nanocomposites as a reusable catalyst for highly efficient reduction of organic pollutants. Catal. Commun. 2019, 126, 35–39. [Google Scholar] [CrossRef]
- Patri, T.; Justin, P.; Babu, P.D.; Ghosh, A. Analysis of dielectric and magnetic phase transitions in Yb(Fe0.5Cr0.5) O3 bulk perovskite. Appl. Phys. A 2019, 125, 224.1–224.12. [Google Scholar] [CrossRef]
- Wei, Y.; Li, H.; Zhang, R.; Xie, H.; Chen, X. Z-scheme CuFe2O4–TiO2 nanocomposite microspheres for the photodegradation of methylene blue. Res. Chem. Intermed. 2018, 44, 7107–7116. [Google Scholar] [CrossRef]
- Dong, Z.; Niu, C.; Guo, H.; Niu, H.; Liang, S.; Liang, C.; Liu, H.Y.; Yang, Y. Anchoring CuFe2O4 nanoparticles into N-doped carbon nanosheets for peroxymonosulfate activation: Built-in electric field dominated radical and non-radical process. Chem. Eng. J. 2021, 426, 130850. [Google Scholar] [CrossRef]
- Taei, M.; Hasanpour, F.; Dinari, M.; Dehghani, E. Au nanoparticles decorated reduced graphene oxide/layered double hydroxide modified glassy. Measurement 2017, 99, 90–97. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Lin, H.; Nong, Q.; Cui, M.; Wu, Y.; He, Y. Fabrication and characterization of hollow CdMoO4 coupled g-C3N4 heterojunction with enhanced photocatalytic activity. J. Hazard. Mater. 2015, 299, 333–342. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Trzci’nski, K.; Łapi´nski, M.; Nowaczyk, G.; Zieli´nska-Jurek, A. UV-Vis-Induced Degradation of Phenol over Magnetic Photocatalysts Modified with Pt, Pd, Cu and Au Nanoparticles. Nanomaterials 2018, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Gao, Y.; Chen, F.; Shi, H. Metallic NiSe cocatalyst decorated g-C3N4 with enhanced photocatalytic activity. Chem. Eng. J. 2020, 413, 127474. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, P.; Zhuo, M.; Wang, F.; Su, Y.; Chen, T.; Yao, K.; Cai, Z.; Lv, W.; Liu, G. Degradation of Indometacin by Simulated Sunlight Activated CDs-loaded BiPO4 Photocatalyst: Roles of oxidative species. Appl. Catal. B 2018, 221, 129–139. [Google Scholar] [CrossRef]
- Shi, H.; Yu, Y.; Zhang, Y.; Feng, X.; Zhao, X.; Tan, H.; Ji, H.; Khan, S.; Li, Y. Polyoxometalate/TiO2/Ag composite nanofibers with enhanced photocatalytic performance under visible light. Appl. Catal. B 2018, 221, 280–289. [Google Scholar] [CrossRef]
- Shi, H.; Fu, J.; Jiang, W.; Wang, Y.; Liu, B.; Liu, J.; Ji, H.; Wang, W.; Chen, Z. Construction of g-C3N4/Bi4Ti3O12 hollow nanofibers with highly efficient visible-light-driven photocatalytic performance. Colloids Surf. A 2021, 615, 126063. [Google Scholar] [CrossRef]
- Dong, X.; Ren, B.; Sun, Z.; Li, C.; Zhang, X.; Kong, M.; Zheng, S.; Dionysiou, D. Monodispersed CuFe2O4 nanoparticles anchored on natural kaolinite as highly efficient peroxymonosulfate catalyst for Bisphenol A degradation. Appl. Catal. B 2019, 217, 253206. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, J.; Niu, S.; Wang, X.; Li, T.; Liu, S.; Lin, Y.; Xie, T.; Dong, S. Comparing dark- and photo-Fenton-like degradation of emerging pollutant over photo-switchable Bi2WO6/CuFe2O4: Investigation on dominant reactive oxidation species. J. Environ. Sci. 2021, 8, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, Y.; Xiao, B.; Wu, X.; Zhang, G. Magnetic yolk-shell structure of ZnFe2O4 nanoparticles for enhanced visible light photo-Fenton degradation towards antibiotics and mechanism study. Appl. Surf. Sci. 2020, 513, 145820. [Google Scholar] [CrossRef]
- Wang, Y.; Song, H.; Chen, J.; Chai, S.; Chen, C.; Wang, Y.; He, C. A novel solar photo-Fenton system with self-synthesizing H2O2: Enhanced photo-induced catalytic performances and mechanism insights. Appl. Surf. Sci. 2020, 512, 145650. [Google Scholar] [CrossRef]
- Han, C.; Park, H.D.; Kim, S.B.; Yargeau, V.V.; Choi, J.W.; Lee, S.H.; Park, J.A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Q.; Liu, P.; Ma, S.; Xie, B.; Yang, K.; Zhao, Y. Novel up-conversion carbon quantum dots/α-FeOOH nanohybrids eliminate tetracycline and its related drug resistance in visible-light responsive Fenton system. Appl. Catal. B-Environ. 2020, 263, 118336. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Kang, L.; Gao, Z.; Ren, F. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: Acceleration of Fe(II)/ Fe(III) cycle under visible light irradiation. Appl. Catal. B-Environ. 2020, 263, 118282. [Google Scholar]
- Zhao, J.; Ji, M.; Di, J.; Zhang, Y.; He, M.; Li, H.; Xia, J. Novel Z-scheme heterogeneous photo-Fenton-like g-C3N4/FeOCl for the pollutants degradation under visible light irradiation. J. Photochem. Photobiol. A 2020, 391, 112343. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, K.; Han, X.; Zhao, Q.; Wang, D.; Fu, F.; Liang, Y. Highly efficient visible-light-driven photo-Fenton catalytic performance over FeOOH/Bi2WO6 composite for organic pollutant degradation. Alloys. Compds. 2020, 816, 152560. [Google Scholar] [CrossRef]
- Li, X.; Zeng, Z.; Zeng, G.; Wang, D.; Xiao, R.; Wang, Y.; Zhou, C.; Yi, H.; Ye, S.; Yang, Y.; et al. A “bottle-around-ship” like method synthesized yolk-shell Ag3PO4@MIL-53(Fe) Z-scheme photocatalysts for enhanced tetracycline removal. J. Colloid Interface Sci. 2020, 561, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gao, J.; Li, T.; Chen, Y.; Wu, Q.; Xie, T.; Lin, Y.; Dong, S. Visible-light-driven photo-Fenton reaction with a-Fe2O3/BiOI at near neutral pH: Boosted photogenerated charge separation, optimum operating parameters and mechanism insight. J. Colloid Interface Sci. 2019, 554, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Mei, Y.; Li, J.; Yao, T.; Yang, Y.; Jia, W.; Tong, X.; Wu, J.; Xin, B. Highly efficient microwave-assisted Fenton degradation of metacycline using pine-needle-like CuCo2O4 nanocatalyst. Chem. Eng. J. 2019, 373, 1158–1167. [Google Scholar] [CrossRef]
- Zhu, G.; Yu, X.; Xie, F.; Feng, W. Ultraviolet light assisted heterogeneous Fenton degradation of tetracycline based on polyhedral Fe3O4 nanoparticles with exposed high-energy {110} facets. Appl. Surf. Sci. 2019, 485, 496–505. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, K.; Han, X.; Zhao, Q.; Wang, D.; Fu, F. 2D In-Plane CuS/Bi2WO6 p-n heterostructures with promoted visible-light-driven Photo-Fenton degradation performance. Nanomaterials 2019, 8, 1151. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S.; et al. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, W.; Kang, F.; Peng, H.; Wen, J. Enhanced photo-Fenton degradation of tetracycline using TiO2-coated α-Fe2O3 core–shell heterojunction. J. Ind. Eng. Chem. 2018, 68, 14–23. [Google Scholar] [CrossRef]
- Du, D.; Shi, W.; Wang, L.; Zhang, J. Yolk-shell structured Fe3O4@void@TiO2 as a photo-Fenton-like catalyst for the extremely efficient elimination of tetracycline. Appl. Catal. B. 2017, 200, 484–492. [Google Scholar] [CrossRef]
- Shetty, K.; Renuka, L.; Nagaswarupa, H.; Nagabhushana, H.; Anantharaju, K.; Rangappa, D.; Prashantha, S.; Ashwini, K. A comparative study on CuFe2O4, ZnFe2O4 and NiFe2O4:Morphology, Impedance and Photocatalytic studies. Mater. Today Proc. 2017, 4, 11806–11815. [Google Scholar]
- Sun, Q.; Wang, X.; Liu, Y.; Xia, S.; Zhao, J. Activation of peroxymonosulfate by a floating oxygen vacancies—CuFe2O4 photocatalyst under visible light for efficient degradation of sulfamethazine. Sci. Total Environ. 2022, 824, 153630. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y. Optimization of photocatalytic degradation of dye wastewater by CuFe2O4/AgBr composite using response surface methodology. Mater. Res. Express. 2018, 6, 036109. [Google Scholar] [CrossRef]
- Li, R.; Cai, M.; Xie, Z.; Zhang, Q.; Zeng, Y.; Liu, H.; Liu, G.; Lv, W. Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B. 2019, 244, 974–982. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Xu, X.; Liu, Z.; Naraginti, S.; Wan, J. Novel magnetically separable tetrahedral Ag3PO4/NrGO/CuFe2O4 photocatalyst for efficient detoxification of 2,4-dichlorophenol. Environ. Res. 2021, 201, 111519. [Google Scholar] [CrossRef] [PubMed]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Yang, J.; Chen, F.; Chen, Z.; Lv, K. Hollow Nanospheres Organized by Ultra-Small CuFe2O4/C Subunits with Efficient Photo-Fenton-like Performance for Antibiotic Degradation and Cr(VI) Reduction. Catalysts 2022, 12, 687. https://doi.org/10.3390/catal12070687
Sun D, Yang J, Chen F, Chen Z, Lv K. Hollow Nanospheres Organized by Ultra-Small CuFe2O4/C Subunits with Efficient Photo-Fenton-like Performance for Antibiotic Degradation and Cr(VI) Reduction. Catalysts. 2022; 12(7):687. https://doi.org/10.3390/catal12070687
Chicago/Turabian StyleSun, Dazhi, Jiayi Yang, Feng Chen, Zhe Chen, and Kangle Lv. 2022. "Hollow Nanospheres Organized by Ultra-Small CuFe2O4/C Subunits with Efficient Photo-Fenton-like Performance for Antibiotic Degradation and Cr(VI) Reduction" Catalysts 12, no. 7: 687. https://doi.org/10.3390/catal12070687
APA StyleSun, D., Yang, J., Chen, F., Chen, Z., & Lv, K. (2022). Hollow Nanospheres Organized by Ultra-Small CuFe2O4/C Subunits with Efficient Photo-Fenton-like Performance for Antibiotic Degradation and Cr(VI) Reduction. Catalysts, 12(7), 687. https://doi.org/10.3390/catal12070687