Combining Ultraviolet Photolysis with In-Situ Electrochemical Oxidation for Degrading Sulfonamides in Wastewater
Abstract
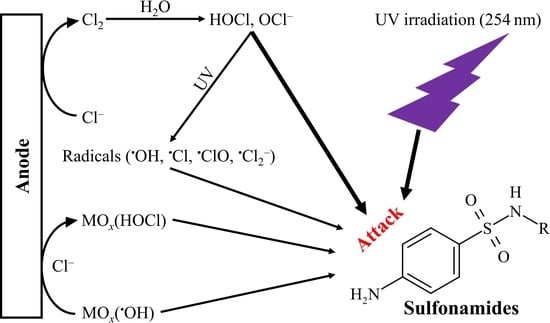
:1. Introduction
2. Results and Discussion
2.1. Influence of Different Aeration Rates
2.2. Influence of Cl− Concentration and Aeration
2.3. Influence of Different Systems and Aeration
2.4. Influence of Photocatalysts
2.5. Influence of Initial pH
2.5.1. Effects of Initial pH and Different Treatments on Sulfonamide Degradation
2.5.2. UV Irradiation Photolysis of Sulfonamides
2.5.3. Electrochemical Oxidation of Sulfonamides
2.5.4. Sulfonamide Degradation by UV Irradiation Combined with Electrochemical Oxidation
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals
3.1.2. Reactor Materials
3.2. Experimental Procedures
3.3. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Wan, Y.; Xie, P.; Wang, Z.; Ding, J.; Wang, J.; Wang, S.; Wiesner, M.R. Comparative study on the pretreatment of algae-laden water by UV/persulfate, UV/chlorine, and UV/H2O2: Variation of characteristics and alleviation of ultrafiltration membrane fouling. Water Res. 2019, 158, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Sichel, C.; Garcia, C.; Andre, K. Feasibility studies: UV/chlorine advanced oxidation treatment for the removal of emerging contaminants. Water Res. 2011, 45, 6371–6380. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.J.; Kronka, M.S.; Fortunato, G.V.; Lanza, M.R.V. Recent advances in electrochemical water technologies for the treatment of antibiotics: A short review. Curr. Opin. Electrochem. 2021, 26, 100674. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Ganzenko, O.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Electrochemical advanced oxidation and biological processes for wastewater treatment: A review of the combined approaches. Environ. Sci. Pollut. Res. 2014, 21, 8493–8524. [Google Scholar] [CrossRef]
- Zhang, S.X.; Pang, X.F.; Yue, Z.; Zhou, Y.H.; Duan, H.T.; Shen, W.J.; Li, J.F.; Liu, Y.L.; Cheng, Q.P. Sulfonamides removed from simulated livestock and poultry breeding wastewater using an in -situ electro-Fenton process powered by photovoltaic energy. Chem. Eng. J. 2020, 397, 125466. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste-water treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Marselli, B.; Garcia-Gomez, J.; Michaud, P.A.; Rodrigo, M.A.; Comninellis, C. Electrogeneration of hydroxyl radicals on boron-doped diamond electrodes. J. Electrochem. Soc. 2003, 150, D79. [Google Scholar] [CrossRef]
- Nowell, L.H.; Hoigne, J. Photolysis of aqueous chlorine at sunlight and ultraviolet wavelengths—2. Hydroxyl radical production. Water Res. 1992, 26, 599–605. [Google Scholar] [CrossRef]
- Malpass, G.R.P.; Miwa, D.W.; Mortari, D.A.; Machado, S.A.S.; Motheo, A.J. Decolorisation of real textile waste using electrochemical techniques: Effect of the chloride concentration. Water Res. 2007, 41, 2969–2977. [Google Scholar] [CrossRef]
- Bonfatti, F.; Ferro, S.; Lavezzo, F.; Malacarne, M.; Lodi, G.; De Battisti, A. Electrochemical incineration of glucose as a model organic substrate. II. Role of active chlorine mediation. J. Electrochem. Soc. 2000, 147, 592. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Waite, T.D. Active chlorine mediated ammonia oxidation revisited: Reaction mechanism, kinetic modelling and implications. Water Res. 2018, 145, 220–230. [Google Scholar] [CrossRef]
- Kishimoto, N.; Katayama, Y.; Kato, M.; Otsu, H. Technical feasibility of UV/electro-chlorine advanced oxidation process and pH response. Chem. Eng. J. 2018, 334, 2363–2372. [Google Scholar] [CrossRef]
- Fang, J.Y.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef]
- Watts, M.J.; Linden, K.G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007, 41, 2871–2878. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous-solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (•OH/•O−) in aqueous-solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Ezazi, M.; Rad, S.V.; Kwon, G. Predicting kinetics of water-rich permeate flux through photocatalytic mesh under visible light illumination. Sci. Rep. 2021, 11, 21065. [Google Scholar] [CrossRef] [PubMed]
- Ezazi, M.; Shrestha, B.; Kim, S.I.; Jeong, B.; Gorney, J.; Hutchison, K.; Lee, D.H.; Kwon, G. Selective Wettability Membrane for Continuous Oil-Water Separation and In Situ Visible Light-Driven Photocatalytic Purification of Water. Glob. Chall. 2020, 4, 2000009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochkodan, V.M.; Rolya, E.A.; Goncharuk, V.V. Photocatalytic membrane reactors for water treatment from organic pollutants. J. Water Chem. Technol. 2009, 31, 227–237. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Chen, X.; Han, Y.; Cao, G. Transformation kinetics and pathways of sulfamonomethoxine by UV/H2O2 in swine wastewater. Chemosphere 2021, 265, 129125. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Feng, M.; Huang, C.-H.; Sun, P. Abiotic transformation and ecotoxicity change of sulfonamide antibiotics in environmental and water treatment processes: A critical review. Water Res. 2021, 202, 117463. [Google Scholar] [CrossRef]
- Chamberlain, E.; Adams, C. Oxidation of sulfonamides, macrolides, and carbadox with free chlorine and monochloramine. Water Res. 2006, 40, 2517–2526. [Google Scholar] [CrossRef]
- Xin, X.; Sun, S.; Zhou, A.; Wang, M.; Song, Y.; Zhao, Q.; Jia, R. Sulfadimethoxine photodegradation in UV-C/H2O2 system: Reaction kinetics, degradation pathways, and toxicity. J. Water Process Eng. 2020, 36, 101293. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H.; Zainal, Z.; Hussein, M.Z. Photocatalytic treatment of 4-chlorophenol in aqueous ZnO suspensions: Intermediates, influence of dosage and inorganic anions. J. Hazard. Mater. 2009, 168, 57–63. [Google Scholar] [CrossRef]
- Sharma, V.K.; Burnett, C.R.; Rivera, W.; Joshi, V.N. Heterogeneous photocatalytic reduction of ferrate(VI) in UV-irradiated titania suspensions. Langmuir 2001, 17, 4598–4601. [Google Scholar] [CrossRef]
- Guo, Z.F.; Ma, R.X.; Li, G.J. Degradation of phenol by nanomaterial TiO2 in wastewater. Chem. Eng. J. 2006, 119, 55–59. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Liu, Y.-L.; Wang, L.; Li, Z.-Y.; Lu, X.-H.; Yang, T.; Ma, J. Enhanced radical generation in an ultraviolet/chlorine system through the addition of TiO2. Environ. Sci. Technol. 2021, 55, 11612–11623. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Qiang, Z.; Li, M.; Bolton, J.R.; Qu, J. UV photolysis kinetics of sulfonamides in aqueous solution based on optimized fluence quantification. Water Res. 2015, 75, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, T. pH significantly affects removal of trace antibiotics in chlorination of municipal wastewater. Water Res. 2012, 46, 3703–3713. [Google Scholar] [CrossRef]
- Zhu, G.; Sun, Q.; Wang, C.; Yang, Z.; Xue, Q. Removal of sulfamethoxazole, sulfathiazole and sulfamethazine in their mixed solution by UV/H2O2 process. Int. J. Environ. Res. Public Health 2019, 16, 1797. [Google Scholar] [CrossRef] [Green Version]
- Baeza, C.; Knappe, D.R.U. Transformation kinetics of biochemically active compounds in low-pressure UV photolysis and UV/H2O2 advanced oxidation processes. Water Res. 2011, 45, 4531–4543. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical fate of sulfa drugs in then aquatic environment: Sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef]
- Yin, R.; Ling, L.; Shang, C. Wavelength-dependent chlorine photolysis and subsequent radical production using UV-LEDs as light sources. Water Res. 2018, 142, 452–458. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Olson, T.M.; Rebenne, L.M. Aqueous chlorination kinetics and mechanism of substituted dihydroxybenzenes. In Water Disinfection and Natural Organic Matter: Characterization and Control; Minear, R.A., Amy, G.L., Eds.; Acs Symposium Series; ACS Publications: Washington, DC, USA, 1996; Volume 649, pp. 48–62. [Google Scholar] [CrossRef]
- Gallard, H.; Von Gunten, U. Chlorination of phenols: Kinetics and formation of chloroform. Environ. Sci. Technol. 2002, 36, 884–890. [Google Scholar] [CrossRef]
- Dodd, M.C.; Huang, C.H. Transformation of the antibacterial agent sulfamethoxazole in reactions with chlorine: Kinetics mechanisms, and pathways. Environ. Sci. Technol. 2004, 38, 5607–5615. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Wu, Z.; Shang, C.; Yao, B.; Hou, S.; Yang, X.; Song, W.; Fang, J. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water. Environ. Sci. Technol. 2017, 51, 10431–10439. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Baum, J.C.; Nesnas, N.; Lee, Y.; Huang, C.-H.; Sharma, V.K. Oxidation of sulfonamide antibiotics of six-membered heterocyclic moiety by ferrate(VI): Kinetics and mechanistic insight into SO2 extrusion. Environ. Sci. Technol. 2019, 53, 2695–2704. [Google Scholar] [CrossRef] [PubMed]











| Sulfonamide | ε × 103 (mol/cm) | k × 103 (min−1) | Reference | ||
|---|---|---|---|---|---|
| Neutral | Anionic | Neutral | Anionic | ||
| SMZ | 16.8 | 20.3 | 1.6 | 3.6 | This study |
| SMM | 17.9 | 24.2 | 3.1 | 9.0 | |
| SMX | 8.8 | 17.2 | 35.4 | 7.1 | |
| SMZ | 17.0 | 21.3 | 0.3 | 1.2 | [34] |
| SMX | 11.5 | 17.1 | 14.5 | 3.8 | |
| Sulfonamide | k × 103 (min−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SMZ | SMM | SMX | |||||||
| UV-E | UV + E | Δ | UV-E | UV + E | Δ | UV-E | UV + E | Δ | |
| pH 5.3 | 6.5 | 7.8 | −1.3 | 9.5 | 10.1 | −0.6 | 24.3 | 26.5 | −2.2 |
| pH 6.3 | 7.2 | 8.6 | −1.4 | 12.3 | 13.4 | −0.9 | 18.7 | 20.3 | −1.6 |
| pH 7.3 | 9.8 | 11.9 | −2.1 | 16.2 | 15.8 | 0.4 | 16.5 | 18.0 | −1.5 |
| pH 8.3 | 12.4 | 13.7 | −1.3 | 17.7 | 16.8 | 0.9 | 16.8 | 16.7 | 0.1 |
| pH 9.3 | 10.7 | 13.4 | −2.7 | 14.9 | 15.2 | 0.3 | 14.8 | 16 | −2.2 |
| Average Δ | - | - | −1.76 | - | - | 0.02 | - | - | −1.48 |
| Sulfonamides | Structure | Chemical Formula | Molecular Weight | pKa [44] |
|---|---|---|---|---|
| Sulfamethazine (SMZ) |  | C12H14N4O2S | 278.33 | pKa1 = 2.30 pKa2 = 7.40 |
| Sulfamonomethoxine (SMM) |  | C11H12N4O3S | 280.30 | pKa1 = 2.00 pKa2 = 6.01 |
| Sulfamethoxazole (SMX) |  | C10H11N3O3S | 253.28 | pKa1 = 2.10 pKa2 = 5.30 |
| Condition | Aeration Rate (L/min) | Current (A) | Cl− Concentration (mg/L) | Initial pH |
|---|---|---|---|---|
| Aeration rate | 0~55 | 1.0 or 1.8 | 20 or 50 | 8.3 |
| Cl− concentration | 0 or 30 | 1.0 | 20~80 | 8.3 |
| System | 0 or 30 | 1.8 | 50 | 8.3 |
| 0 | ||||
| 1.8 | ||||
| Photocatalysts | 30 | 1.8 | 50 | 8.3 |
| 0 | ||||
| Initial pH | 0 | 1.8 | 50 | 5.3~8.3 |
| 0 | ||||
| 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Yuan, J.; Jiang, X.; Han, G.; Tao, Y.; Wu, X. Combining Ultraviolet Photolysis with In-Situ Electrochemical Oxidation for Degrading Sulfonamides in Wastewater. Catalysts 2022, 12, 711. https://doi.org/10.3390/catal12070711
Zheng Z, Yuan J, Jiang X, Han G, Tao Y, Wu X. Combining Ultraviolet Photolysis with In-Situ Electrochemical Oxidation for Degrading Sulfonamides in Wastewater. Catalysts. 2022; 12(7):711. https://doi.org/10.3390/catal12070711
Chicago/Turabian StyleZheng, Zhijie, Julin Yuan, Xinwei Jiang, Gang Han, Yufang Tao, and Xiaogang Wu. 2022. "Combining Ultraviolet Photolysis with In-Situ Electrochemical Oxidation for Degrading Sulfonamides in Wastewater" Catalysts 12, no. 7: 711. https://doi.org/10.3390/catal12070711
APA StyleZheng, Z., Yuan, J., Jiang, X., Han, G., Tao, Y., & Wu, X. (2022). Combining Ultraviolet Photolysis with In-Situ Electrochemical Oxidation for Degrading Sulfonamides in Wastewater. Catalysts, 12(7), 711. https://doi.org/10.3390/catal12070711