Photo–Redox Properties of –SO3H Functionalized Metal-Free g-C3N4 and Its Application in the Photooxidation of Sunset Yellow FCF and Photoreduction of Cr (VI)
Abstract
:1. Introduction
2. Results and Discussion
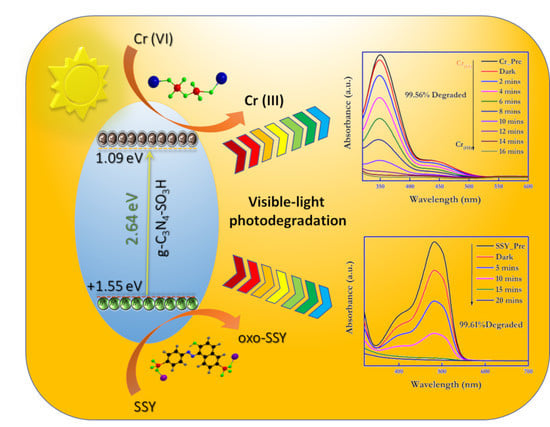
2.1. Photocatalytic Reduction of Cr (VI) and Oxidation of SSY
2.2. The Possible Mechanism for the Photoreduction of Cr (VI) and Photooxidation of SSY
2.3. Influence of pH and Scavenging Species and Recycling Stability
2.4. Comparative Study of the Photodegradation of Cr (VI) and SSY
2.5. Photoelectrochemical Measurements
3. Materials and Methods
3.1. Materials
3.2. Characterizations
3.3. Synthesis of Pristine g-C3N4
3.4. Synthesis of Sulfonic Group-Functionalized g-C3N4
3.5. Photocatalytic Redox Degradation, Electrochemical, and PEC Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Lunk, H.J. Discovery, Properties and Applications of Chromium and Its Compounds. ChemTexts 2015, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Basaran, B.; Ulas, M.; Bitlisli, B.O.; Asian, A. Distribution of Cr (III) and Cr (VI) in Chrome Tanned Leather. Indian J. Chem. Technol. 2008, 15, 511–514. [Google Scholar]
- Rhodes, M.C.; Hébert, C.D.; Herbert, R.A.; Morinello, E.J.; Roycroft, J.H.; Travlos, G.S.; Abdo, K.M. Absence of Toxic Effects in F344/N Rats and B6C3F 1 Mice Following Subchronic Administration of Chromium Picolinate Monohydrate. Food Chem. Toxicol. 2005, 43, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Oginawati, K.; Susetyo, S.H.; Rosalyn, F.A.; Kurniawan, S.B.; Abdullah, S.R.S. Risk Analysis of Inhaled Hexavalent Chromium (Cr6+) Exposure on Blacksmiths from Industrial Area. Environ. Sci. Pollut. Res. 2021, 28, 14000–14008. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.A.; Salem, H. The Toxicology of Chromium with Respect to Its Chemical Speciation: A Review. J. Appl. Toxicol. 1993, 13, 217–224. [Google Scholar] [CrossRef]
- Ray, R.R. Adverse Hematological Effects of Hexavalent Chromium: An Overview. Interdiscip. Toxicol. 2016, 9, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health Hazards of Hexavalent Chromium (Cr (VI)) and Its Microbial Reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef] [PubMed]
- Lacstusu, R. Appraising Levels of Soil Contamination y Pollution with Heavy Metals. European Soil Bureau. Int. Ref. J. Eng. Sci. 1998, 3, 393–399. [Google Scholar]
- Aneyo, I.A.; Doherty, F.V.; Adebesin, O.A.; Hammed, M.O. Biodegradation of Pollutants in Waste Water from Pharmaceutical, Textile and Local Dye Effluent in Lagos, Nigeria. J. Health Pollut. 2016, 6, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moffat, I.; Martinova, N.; Seidel, C.; Thompson, C.M. Hexavalent Chromium in Drinking Water. J. Am. Water Works Assoc. 2018, 110, E22–E35. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z.; Włosek, R. The Influence of Old Leather Tannery District on Chromium Contamination of Soils, Water and Plants. Nat. Sci. 2013, 5, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, R.T.; Bani Mfarrej, M.F.; Alam, P.; Rinklebe, J.; Ahmad, P. Accumulation of Chromium in Plants and Its Repercussion in Animals and Humans. Environ. Pollut. 2022, 301, 119044. [Google Scholar] [CrossRef]
- Stern, C.M.; Jegede, T.O.; Hulse, V.A.; Elgrishi, N. Electrochemical Reduction of Cr(vi) in Water: Lessons Learned from Fundamental Studies and Applications. Chem. Soc. Rev. 2021, 50, 1642–1667. [Google Scholar] [CrossRef]
- Valari, M.; Antoniadis, A.; Mantzavinos, D.; Poulios, I. Photocatalytic Reduction of Cr(VI) over Titania Suspensions. Catal. Today 2015, 252, 190–194. [Google Scholar] [CrossRef]
- Kobylewski, S.; Jacobson, M.F. Toxicology of Food Dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef]
- Basu, A.; Kumar, G.S. Interaction of Toxic Azo Dyes with Heme Protein: Biophysical Insights into the Binding Aspect of the Food Additive Amaranth with Human Hemoglobin. J. Hazard. Mater. 2015, 289, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Merinas-Amo, R.; Martínez-Jurado, M.; Jurado-Güeto, S.; Alonso-Moraga, Á.; Merinas-Amo, T. Biological Effects of Food Coloring in in Vivo and in Vitro Model Systems. Foods 2019, 8, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandir, D. DNA Damage in Human Germ Cell Exposed to the Some Food Additives in Vitro. Cytotechnology 2016, 68, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Algarni, A.A. In Vitro Effects of Sunset Yellow on Chromosomal Damage and Sister Chromatid Exchanges in Human Peripheral Lymphocytes. Annu. Res. Rev. Biol. 2021, 36, 88–94. [Google Scholar] [CrossRef]
- El-Borm, H.; Badawy, G.; Hassab El-Nabi, S.; El-Sherif, W.; Atallah, M. Toxicity of Sunset Yellow Fcf and Tartrazine Dyes on Dna and Cell Cycle of Liver and Kidneys of the Chick Embryo: The Alleviative Effects of Curcumin. Egypt. J. Zool. 2020, 74, 43–55. [Google Scholar] [CrossRef]
- Colakoglu, F.; Selcuk, M.L. The Embryotoxic Effects of in Ovo Administered Sunset Yellow FCF in Chick Embryos. Vet. Sci. 2021, 8, 31. [Google Scholar] [CrossRef]
- Bastaki, M.; Mendes, O.R.; Bauter, M.R.; Taylor, S.V. Assessment of FD&C Yellow No. 6 (Sunset Yellow FCF) Effects on Sperm Count, Motility and Viability in the Rat in a 28-Day Toxicity Study. Regul. Toxicol. Pharmacol. 2019, 108, 104479. [Google Scholar] [CrossRef]
- Hassan, G.M. Effects of Some Synthetic Coloring Additives on DNA Damage and Chromosomal Aberrations of Rats. Arab J. Biotechnol. 2010, 13, 13–24. [Google Scholar]
- Joshi, V.; Pancharatna, K. Food Colorant Sunset Yellow (E110) Intervenes Developmental Profile of Zebrafish (Danio Rerio). J. Appl. Toxicol. 2019, 39, 571–581. [Google Scholar] [CrossRef]
- Verma, S.K.; Khandegar, V.; Saroha, A.K. Removal of Chromium from Electroplating Industry Effluent Using Electrocoagulation. J. Hazard. Toxic Radioact. Waste 2013, 17, 146–152. [Google Scholar] [CrossRef]
- Mnif, A.; Bejaoui, I.; Mouelhi, M.; Hamrouni, B. Hexavalent Chromium Removal from Model Water and Car Shock Absorber Factory Effluent by Nanofiltration and Reverse Osmosis Membrane. Int. J. Anal. Chem. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Korak, J.A.; Huggins, R.; Arias-Paic, M. Regeneration of Pilot-Scale Ion Exchange Columns for Hexavalent Chromium Removal. Water Res. 2017, 118, 141–151. [Google Scholar] [CrossRef]
- Basumatary, A.K.; Kumar, R.V.; Ghoshal, A.K.; Pugazhenthi, G. Cross Flow Ultrafiltration of Cr (VI) Using MCM-41, MCM-48 and Faujasite (FAU) Zeolite-Ceramic Composite Membranes. Chemosphere 2016, 153, 436–446. [Google Scholar] [CrossRef]
- Shin, J.; Bae, S.; Chon, K. Fenton Oxidation of Synthetic Food Dyes by Fe-Embedded Coffee Biochar Catalysts Prepared at Different Pyrolysis Temperatures: A Mechanism Study. Chem. Eng. J. 2021, 421, 129943. [Google Scholar] [CrossRef]
- Sarwade, V.D. Bio-Catalytic Action of Pseudomonas DL17 on Environmental Contaminant Sunset Yellow FCF. Int. J. Res. Appl. Sci. Eng. Technol. 2020, 8, 1056–1064. [Google Scholar] [CrossRef]
- Moghaddam, M.A.; Seyyedi, K. Optimization of the Sunset Yellow Dye Removal by Electrocoagulation Using a Response Surface Method. Water Sci. Technol. 2022, 85, 206–219. [Google Scholar] [CrossRef]
- Yayayürük, O.; Erdem Yayayürük, A.; Özmen, P.; Karagöz, B. PDMAEMA Grafted Microspheres as an Efficient Adsorbent for the Removal of Sunset Yellow from Pharmaceutical Preparations, Beverages and Waste Water. Eur. Polym. J. 2020, 141, 110089. [Google Scholar] [CrossRef]
- Ahmad, Z.U.; Yao, L.; Wang, J.; Gang, D.D.; Islam, F.; Lian, Q.; Zappi, M.E. Neodymium Embedded Ordered Mesoporous Carbon (OMC) for Enhanced Adsorption of Sunset Yellow: Characterizations, Adsorption Study and Adsorption Mechanism. Chem. Eng. J. 2019, 359, 814–826. [Google Scholar] [CrossRef]
- Kannan, A.; Venkatesvaran, H.; Ananthakrishnan, D.; Mayavan, A.; Gandhi, S. An Early Detection of Prostate Cancer Drug in Water to Prevent Loss of Biodiversity. Process Saf. Environ. Prot. 2021, 151, 51–62. [Google Scholar] [CrossRef]
- Venkatesvaran, H.; Kannan, A.; Mayavan, A.; Dhanabal, A.; Gandhi, S. Metal Nanoparticle Ornated Mesoporous Silica: A Potential Nano-Interface for Uric Acid Detection. Microporous Mesoporous Mater. 2021, 324, 111313. [Google Scholar] [CrossRef]
- Balu, S.; Chen, Y.-L.; Chen, S.-W.; Yang, T.C.-K. Rational Synthesis of BixFe1–XVO4 Heterostructures Impregnated Sulfur-Doped g-C3N4: A Visible-Light-Driven Type-II Heterojunction Photo(Electro)Catalyst for Efficient Photodegradation of Roxarsone and Photoelectrochemical OER Reactions; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 8862277121. [Google Scholar]
- Xu, H.; Hao, Z.; Feng, W.; Wang, T.; Fu, X. The Floating Photocatalytic Spheres Loaded with Weak Light-Driven TiO2-Based Catalysts for Photodegrading Tetracycline in Seawater. Mater. Sci. Semicond. Process. 2022, 144, 106610. [Google Scholar] [CrossRef]
- Velasco, G.; Gutiérrez-Granados, S.; Ponce De León, C.; Alatorre, A.; Walsh, F.C.; Rodríguez-Torres, I. The Electrochemical Reduction of Cr(VI) Ions in Acid Solution at Titanium and Graphite Electrodes. J. Environ. Chem. Eng. 2016, 4, 3610–3617. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Chen, P.; Nie, W.; Xu, Y.; Zhou, Y. Improved Photocatalytic Reduction of Cr(VI) by Molybdenum Disulfide Modified with Conjugated Polyvinyl Alcohol. Chem. Eng. J. 2019, 359, 1205–1214. [Google Scholar] [CrossRef]
- Pavasaryte, L.; Balu, S.; Yang, T.C.K. Synthesis of Sol–Gel Derived Holmium Aluminium Garnet on Exfoliated g-C3N4: A Novel Visible-Light-Driven Z-Scheme Photocatalyst for the Degradation of Sunset Yellow FCF. J. Mater. Sci. Mater. Electron. 2019, 30, 20132–20143. [Google Scholar] [CrossRef]
- Heymann, L.; Schiller, B.; Noei, H.; Stierle, A.; Klinke, C. A New Synthesis Approach for Carbon Nitrides: Poly(Triazine Imide) and Its Photocatalytic Properties. ACS Omega 2018, 3, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A Fantastic Graphitic Carbon Nitride (g-C3N4) Material: Electronic Structure, Photocatalytic and Photoelectronic Properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Balu, S.; Chen, Y.L.; Juang, R.C.; Yang, T.C.K.; Juan, J.C. Morphology-Controlled Synthesis of α–Fe2O3 Nanocrystals Impregnated on g-C3N4–SO3H with Ultrafast Charge Separation for Photoreduction of Cr (VI) Under Visible Light. Environ. Pollut. 2020, 267, 115491. [Google Scholar] [CrossRef]
- Balu, S.; Chen, Y.L.; Yang, T.C.K.; Chen, J.N.; Chen, S.W. Effect of Ultrasound-Induced Hydroxylation and Exfoliation on P90–TiO2/g-C3N4 Hybrids with Enhanced Optoelectronic Properties for Visible-Light Photocatalysis and Electrochemical Sensing. Ceram. Int. 2020, 46, 18002–18018. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, Y.; Cong, B.; Xing, W.; Chen, G. Enhanced Carriers Separation Efficiency in G-C3N4 Modified with Sulfonic Groups for Efficient Photocatalytic Cr(VI) Reduction. Mater. Res. Bull. 2020, 122, 110681. [Google Scholar] [CrossRef]
- Patnaik, S.; Martha, S.; Madras, G.; Parida, K. The Effect of Sulfate Pre-Treatment to Improve the Deposition of Au-Nanoparticles in a Gold-Modified Sulfated g-C3N4 Plasmonic Photocatalyst towards Visible Light Induced Water Reduction Reaction. Phys. Chem. Chem. Phys. 2016, 18, 28502–28514. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Shi, J.; Liang, Y.; Han, J.; Tong, Y.; Li, W. Novel SPVA/g-C3N4-SA/PAN Pervaporation Membranes with Porous Catalytic Layers for Esterification Enhancement. Ind. Eng. Chem. Res. 2021, 60, 6089–6100. [Google Scholar] [CrossRef]
- Majdoub, M.; Anfar, Z.; Amedlous, A. Emerging Chemical Functionalization of G-C3N4: Covalent/Noncovalent Modifications and Applications. ACS Nano 2020, 14, 12390–12469. [Google Scholar] [CrossRef]
- Zhao, M.; Pan, Z.; Chen, Q.; Zhou, H. Catalytic Alcoholysis to Prepare Diosgenin with a Solid Acid Based on Nano TiO2. Catal. Lett. 2022, 152, 1–12. [Google Scholar] [CrossRef]
- Choudhary, P.; Sen, A.; Kumar, A.; Dhingra, S.; Nagaraja, C.M.; Krishnan, V. Sulfonic Acid Functionalized Graphitic Carbon Nitride as Solid Acid–Base Bifunctional Catalyst for Knoevenagel Condensation and Multicomponent Tandem Reactions. Mater. Chem. Front. 2021, 5, 6265–6278. [Google Scholar] [CrossRef]
- Qian, X.B.; Peng, W.; Huang, J.H. Fluorescein-Sensitized Au/g-C3N4 Nanocomposite for Enhanced Photocatalytic Hydrogen Evolution under Visible Light. Mater. Res. Bull. 2018, 102, 362–368. [Google Scholar] [CrossRef]
- Nanda, B.; Pradhan, A.C.; Parida, K.M. Fabrication of Mesoporous CuO/ZrO2-MCM-41 Nanocomposites for Photocatalytic Reduction of Cr(VI). Chem. Eng. J. 2017, 316, 1122–1135. [Google Scholar] [CrossRef] [Green Version]
- Nezar, S.; Cherifi, Y.; Barras, A.; Addad, A.; Dogheche, E.; Saoula, N.; Laoufi, N.A.; Roussel, P.; Szunerits, S.; Boukherroub, R. Efficient Reduction of Cr(VI) under Visible Light Irradiation Using CuS Nanostructures. Arab. J. Chem. 2019, 12, 215–224. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Y.; Zhou, M.; Li, Z.; Xu, S.; Yao, C. Facile Synthesis of Novel ZnFe2O4/CdS Nanorods Composites and Its Efficient Photocatalytic Reduction of Cr(VI) under Visible-Light Irradiation. J. Ind. Eng. Chem. 2018, 58, 64–73. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Jin, Q.; Ren, Z.; Sun, Y.; Zhang, R.; Zhai, Y.; Liu, Y. Photocatalytic Reduction of Cr (VI) on Nano-Sized Red Phosphorus under Visible Light Irradiation. J. Colloid Interface Sci. 2019, 537, 256–261. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, X.; Xu, J.; Crittenden, J.C.; Liu, E.; Zhang, Y.; Cong, Y. Highly Enhanced Photocatalytic Reduction of Cr(VI) on AgI/TiO2 under Visible Light Irradiation: Influence of Calcination Temperature. J. Hazard. Mater. 2016, 307, 213–220. [Google Scholar] [CrossRef]
- Liu, L.; Luo, C.; Xiong, J.; Yang, Z.; Zhang, Y.; Cai, Y.; Gu, H. Reduced Graphene Oxide (RGO) Decorated TiO2microspheres for Visible-Light Photocatalytic Reduction of Cr(VI). J. Alloys Compd. 2017, 690, 771–776. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Dhatshanamurthi, P.; Shanthi, M. Preparation and Characterization of SeO2/TiO2 Composite Photocatalyst with Excellent Performance for Sunset Yellow Azo Dye Degradation under Natural Sunlight Illumination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 489–498. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic Degradation of an Azo Dye Sunset Yellow under UV-A Light Using TiO2/CAC Composite Catalysts. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 100–108. [Google Scholar] [CrossRef]
- Hassanien, R.; Abed-Elmageed, A.A.I.; Husein, D.Z. Eco-Friendly Approach to Synthesize Selenium Nanoparticles: Photocatalytic Degradation of Sunset Yellow Azo Dye and Anticancer Activity. ChemistrySelect 2019, 4, 9018–9026. [Google Scholar] [CrossRef]
















| S. No. | Material/ | Contaminant | Concentration | Duration | Reference |
|---|---|---|---|---|---|
| Composite | |||||
| 1. | ZnFe2O4/CdS | Cr (VI) | 100 ppm | 120 min | [56] |
| 2. | CPVA/MoS2-OH | Cr (VI) | 25–75 ppm | 105 min | [40] |
| 3. | Nano-sized red phosphorus | Cr (VI) | 20 ppm | 36 min | [57] |
| 4. | AgI/TiO2 | Cr (VI) | 160 µmol/ | 60 min | [58] |
| 47 ppm | |||||
| 5. | TiO2-P25 | Cr (VI) | 10 ppm | 60 min | [15] |
| 6. | TiO2-rGO | Cr (VI) | 10 ppm | 180 min | [59] |
| 7. | g-C3N4-SO3H | Cr (VI) | 100 ppm | 16 min | This work |
| 8. | SeO2/TiO2 | SSY | 120 ppm | 90 min | [60] |
| 9. | HoAG/g-C3N4 | SSY | 5 ppm | 60 min | [41] |
| 10. | CAC/TiO2 | SSY | 300 µmol/ | 80 min | [61] |
| 135.7 ppm | |||||
| 11. | Se NPLs | SSY | 5 ppm | 600 min | [62] |
| 12. | g-C3N4-SO3H | SSY | 50 ppm | 20 min | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesvaran, H.; Balu, S.; Chowdhury, A.; Chen, S.-W.; Yang, T.C.-K. Photo–Redox Properties of –SO3H Functionalized Metal-Free g-C3N4 and Its Application in the Photooxidation of Sunset Yellow FCF and Photoreduction of Cr (VI). Catalysts 2022, 12, 751. https://doi.org/10.3390/catal12070751
Venkatesvaran H, Balu S, Chowdhury A, Chen S-W, Yang TC-K. Photo–Redox Properties of –SO3H Functionalized Metal-Free g-C3N4 and Its Application in the Photooxidation of Sunset Yellow FCF and Photoreduction of Cr (VI). Catalysts. 2022; 12(7):751. https://doi.org/10.3390/catal12070751
Chicago/Turabian StyleVenkatesvaran, Harikrishnan, Sridharan Balu, Anuradha Chowdhury, Shih-Wen Chen, and Thomas C.-K. Yang. 2022. "Photo–Redox Properties of –SO3H Functionalized Metal-Free g-C3N4 and Its Application in the Photooxidation of Sunset Yellow FCF and Photoreduction of Cr (VI)" Catalysts 12, no. 7: 751. https://doi.org/10.3390/catal12070751
APA StyleVenkatesvaran, H., Balu, S., Chowdhury, A., Chen, S. -W., & Yang, T. C. -K. (2022). Photo–Redox Properties of –SO3H Functionalized Metal-Free g-C3N4 and Its Application in the Photooxidation of Sunset Yellow FCF and Photoreduction of Cr (VI). Catalysts, 12(7), 751. https://doi.org/10.3390/catal12070751