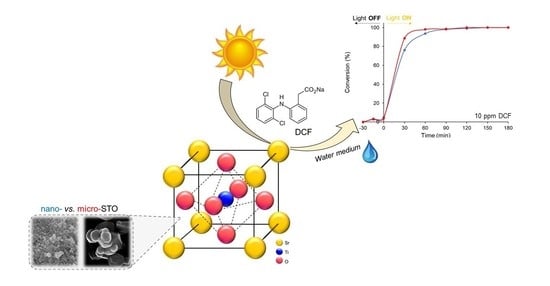
Sustainable Solar Light Photodegradation of Diclofenac by Nano- and Micro-Sized SrTiO3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalyst Characterization
2.2. Diclofenac Abatement
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Diclofenac Abatement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Sophia, C.A.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts 2021, 11, 562. [Google Scholar] [CrossRef]
- Djellabi, R.; Giannantonio, R.; Falletta, E.; Bianchi, C.L. SWOT analysis of photocatalytic materials towards large scale environmental remediation. Curr. Opin. Chem. Eng. 2021, 33, 100696. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z. Industrial water pollution, water environment treatment, and health risks in China. Environ. Pollut. 2016, 218, 358–365. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization & United Nations Children’s Fund (UNICEF). Progress on Sanitation and Drinking Water—2015 Update and MDG Assessment. 2015. Available online: https://apps.who.int/iris/handle/10665/177752 (accessed on 15 July 2022).
- Bianchi, C.L.; Djellabi, R.; Della Pina, C.; Falletta, E. Doped-polyaniline based sorbents for the simultaneous removal of heavy metals and dyes from water: Unravelling the role of synthesis method and doping agent. Chemosphere 2022, 286, 131941. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Saber, A.N.; Djellabi, R.; Fellah, I.; Abderrahim, N.; Bianchi, C.L. Synergistic sorption/photo-Fenton removal of typical substituted and parent polycyclic aromatic hydrocarbons from coking wastewater over CuO-Montmorillonite. J. Water Process Eng. 2021, 44, 102377. [Google Scholar] [CrossRef]
- Meroni, D.; Bianchi, C.L.; Boffito, D.C.; Cerrato, G.; Bruni, A.; Sartirana, M.; Falletta, E. Piezo-enhanced photocatalytic diclofenac mineralization over ZnO. Ultrason. Sonochem. 2021, 75, 105615. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. Int. J. Environ. Res. Public Health 2019, 17, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerrato, G.; Bianchi, C.L.; Galli, F.; Pirola, C.; Morandi, S.; Capucci, V. Micro-TiO2 coated glass surfaces safely abate drugs in surface water. J. Hazard. Mater. 2019, 363, 328–334. [Google Scholar] [CrossRef]
- Stucchi, M.; Cerrato, G.; Bianchi, C.L. Ultrasound to improve both synthesis and pollutants degradation based on metal nanoparticles supported on TiO2. Ultrason. Sonochem. 2019, 51, 462–468. [Google Scholar] [CrossRef]
- Schieppati, D.; Galli, F.; Peyot, M.-L.; Yargeau, V.; Bianchi, C.L.; Boffito, D.C. An ultrasound-assisted photocatalytic treatment to remove an herbicidal pollutant from wastewaters. Ultrason. Sonochem. 2019, 54, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Meroni, D.; Jiménez-Salcedo, M.; Falletta, E.; Bresolin, B.M.; Kait, C.F.; Boffito, D.C.; Bianchi, C.; Pirola, C. Sonophotocatalytic degradation of sodium diclofenac using low power ultrasound and micro sized TiO2. Ultrason. Sonochem. 2020, 67, 105123. [Google Scholar] [CrossRef]
- Kavitha, S.; Ranjith, R.; Jayamani, N.; Vignesh, S.; Palanivel, B.; Djellabi, R.; Bianchi, C.L.; Alharthi, F.A. Fabrication of visible-light-responsive TiO2/α-Fe2O3-heterostructured composite for rapid photo-oxidation of organic pollutants in water. J. Mater. Sci. Mater. Electron. 2022, 33, 8906–8919. [Google Scholar] [CrossRef]
- Monllor-Satoca, D.; Bonete, P.; Djellabi, R.; Cerrato, G.; Operti, L.; Gomez, R.; Bianchi, C.L. Comparative Photo-Electrochemical and Photocatalytic Studies with Nanosized TiO2 Photocatalysts towards Organic Pollutants Oxidation. Catalysts 2021, 11, 349. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent Progress on Titanium Dioxide Nanomaterials for Photocatalytic Applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of photocatalytic activity of titanium dioxide using non-metal doping methods under visible light: A review. Int. J. Environ. Sci. Technol. 2018, 15, 2009–2032. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.L.; Argirusis, C.; Pifferi, V.; Neppolian, B.; Cerrato, G.; Boffito, D.C. Ultrasound assisted synthesis of Ag-decorated TiO2 active in visible light. Ultrason. Sonochem. 2018, 40, 282–288. [Google Scholar] [CrossRef]
- Stucchi, M.; Boffito, D.C.; Pargoletti, E.; Cerrato, G.; Bianchi, C.L.; Cappelletti, G. Nano-MnO2 Decoration of TiO2 Microparticles to Promote Gaseous Ethanol Visible Photoremoval. Nanomaterials 2018, 8, 686. [Google Scholar] [CrossRef] [Green Version]
- Falletta, E.; Bruni, A.; Sartirana, M.; Boffito, D.C.; Cerrato, G.; Giordana, A.; Djellabi, R.; Khatibi, E.S.; Bianchi, C.L. Solar Light Photoactive Floating Polyaniline/TiO2 Composites for Water Remediation. Nanomaterials 2021, 11, 3071. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I. Study of phase composition, photocatalytic activity, and photoluminescence of TiO2 with Eu additive produced by the extraction-pyrolytic method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Kumarov, A.; Atabaev, T.S.; Hapeshi, E.; Vakros, J.; Mantzavinos, D.; Poulopoulos, S.G. Degradation of 4-Tert-Butylphenol in Water Using Mono-Doped (M1: Mo, W) and Co-Doped (M2-M1: Cu, Co, Zn) Titania Catalysts. Nanomaterials 2022, 12, 2326. [Google Scholar] [CrossRef]
- Wu, X.; Lin, J.; Xu, Z.; Zhao, C.; Lin, C.; Wang, H.; Lin, T.; Zheng, X.; Sa, B.; Zhang, Q.; et al. Defect Management and Multi-Mode Optoelectronic Manipulations via Photo-Thermochromism in Smart Windows. Laser Photonics Rev. 2021, 15, 2100211. [Google Scholar] [CrossRef]
- Shi, L.; Chen, K.; Zhai, A.; Li, G.; Fan, M.; Hao, Y.; Zhu, F.; Zhang, H.; Cui, Y. Status and Outlook of Metal–Inorganic Semiconductor–Metal Photodetectors. Laser Photonics Rev. 2020, 15, 2000401. [Google Scholar] [CrossRef]
- Yang, B.; Wang, M.; Hu, X.; Zhou, T.; Zang, Z. Highly efficient semitransparent CsPbIBr2 perovskite solar cells via low-temperature processed In2S3 as electron-transport-layer. Nano Energy 2019, 57, 718–727. [Google Scholar] [CrossRef]
- da Silva, L.F.; Lopes, O.F.; de Mendonça, V.R.; Clavalho, K.T.G.; Longo, E.; Ribeiro, C.; Mastelaro, V.R. An understanding of the photocatalytic properties and pollutant degradation mechanism of SrTiO3 nanoparticles. Photochem. Photobiol. 2016, 92, 371–378. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, M.; Shi, Q.; Wen, F.; Liu, L.; Dong, B.; Haroun, A.; Yang, Y.; Vachon, P.; Guo, X.; et al. Progress in TENG technology—A journey from energy harvesting to nanoenergy and nanosystem. EcoMat 2020, 2, e12058. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, H.; Qi, M.; Shen, J.; Liu, W.; Guo, E.-J.; Li, D.; Zhang, Y.; Wu, Z. Enhanced Upconversion Photoluminescence Assisted by Flexoelectric Field in Oxide Nanomembranes. Laser Photonics Rev. 2022, 16, 2100454. [Google Scholar] [CrossRef]
- Eglitis, R.I.; Purans, J.; Popov, A.I.; Jia, R. Systematic trends in YAlO3, SrTiO3, BaTiO3, BaZrO3 and (111) surface ab initio calculations. Int. J. Mod. Phys. B 2019, 33, 1950390. [Google Scholar] [CrossRef]
- Chang, C.-W.; Hu, C. Graphene oxide-derived carbon-doped SrTiO3 for highly efficient photocatalytic degradation of organic pollutants under visible light irradiation. Chem. Eng. J. 2020, 383, 123116. [Google Scholar] [CrossRef]
- Hu, C.; Huang, H.-X.; Lin, Y.-F.; Yoshida, M.; Chen, T.-H. Decoration of SrTiO3 nanofibers by BiOI for photocatalytic methyl orange degradation under visible light irradiation. J. Taiwan Inst. Chem. Eng. 2019, 96, 264–272. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Krishnan, V. Perovskite Oxide Based Materials for Energy and Environment-Oriented Photocatalysis. ACS Catal. 2020, 10, 10253–10315. [Google Scholar] [CrossRef]
- Kong, J.; Yang, T.; Rui, Z.; Ji, H. Perovskite-based photocatalysts for organic contaminants removal: Current status and future perspectives. Catal. Today 2019, 327, 47–63. [Google Scholar] [CrossRef]
- Djellabi, R.; Ordonez, M.F.; Conte, F.; Falletta, E.; Bianchi, C.L.; Rossetti, I. A review of advances in multifunctional XTiO3 perovskite-type oxides as piezo-photocatalysts for environmental remediation and energy production. J. Hazard. Mater. 2022, 421, 126792. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorg. Chem. Commun. 2020, 120, 108140. [Google Scholar] [CrossRef]
- Aliste, M.; Garrido, I.; Flores, P.; Hellin, P.; Vela, N.; Navarro, S.; Fenoll, J. Reclamation of agro-wastewater polluted with thirteen pesticides by solar photocatalysis to reuse in irrigation of greenhouse lettuce grown. J. Environ. Manage. 2020, 266, 110565. [Google Scholar] [CrossRef] [PubMed]
- Kushniarou, A.; Garrido, I.; Fenoll, J.; Vela, N.; Flores, P.; Navarro, G.; Hellin, P.; Navarro, S. Solar photocatalytic reclamation of agro-waste water polluted with twelve pesticides for agricultural reuse. Chemosphere 2019, 214, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Arnab, D.; Rituparna, B.; Ajeet, K.; Subho, M. Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: New Delhi, India, 2014. [Google Scholar]
- UNEP. The Snapshot Report of the World’s Water Quality; UNEP: Nairobi, Kenya, 2016. [Google Scholar]
- Iovino, P.; Chianese, S.; Canzano, S.; Pisciandaro, M.; Musmarra, D. Photodegradation of diclofenac in wastewaters. Desalin. Water Treatment 2017, 61, 293–297. [Google Scholar]
- Calza, P.; Sakkas, V.A.; Villioti, A.; Massolino, C.; Boti, V.; Pelizzetti, E.; Albanis, T. Multivariate experimental design for the photocatalytic degradation of imipramine, determination of the reaction pathway and identification of intermediate products. Appl. Catal. B Environ. 2008, 84, 379–388. [Google Scholar] [CrossRef]
- Wiegel, S.; Aulinger, A.; Brockmeyer, R.; Harms, H.; Löffler, J.; Reincke, H. Pharmaceuticals in the river Elbe and its tributaries. Chemosphere 2004, 57, 107–126. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Radjenovic, J.; Petrovic, M.; Barceló, D. Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal. Bioanal. Chem. 2007, 387, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Vieno, N.M.; Harkki, H.; Tuhkanen, T.; Kronberg, L. Occurrence of pharmaceuticalsin river water and their elimination in a pilot-scale drinking water treatment plant. Environ. Sci. Technol. 2007, 41, 5077–5084. [Google Scholar] [CrossRef]
- Hartmann, J.; Bartels, P.; Mau, U.; Witter, M. Degradation of the drug diclofenac in water by sonolysis in presence of catalysts. Chemosphere 2008, 70, 453–461. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abou-Gamra, Z.M.; Medien, H.A.A.; Hamza, M.A. Effect of porphyrin on photocatalytic activity of TiO2 nanoparticles toward Rhodamine B photodegradation. J. Photochem. Photobiol. B Biol. 2017, 176, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2013/39/EU of the european parliament and of the council. Off. J. Eur. Union 2013, 226, 1–17. [Google Scholar]
- Rodrigues, A.S.; Silveira, J.E.; Carbajo, J.; Zazo, J.A.; Casas, J.A.; Fernandes, A.; Pacheco, M.J.; Ciriaco, L.; Lopes, A. Diclofenac photodegradation with the Perovskites BaFeyTi1-yO3 as catalysts. Environ. Sci. Pollut. Res. 2021, 28, 23822–23832. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M. Electrospinning preparation of NiO/ZnO composite nanofibers for photodegradation of binary mixture of rhodamine B and methylene blue in aqueous solution: Central composite optimization. Appl. Organomet. Chem. 2018, 32, e4335. [Google Scholar] [CrossRef]
- Gunture; Singh, A.; Bhati, A.; Khare, P.; Tripathi, K.M.; Sonkar, S.K. Soluble Graphene Nanosheets for the Sunlight-Induced Photodegradation of the Mixture of Dyes and its Environmental Assessment. Sci. Rep. 2019, 9, 2522. [Google Scholar] [CrossRef]
- International Organization for Standardization. Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. ISO 9277:2. Available online: www.iso.org (accessed on 10 July 2022).
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure App. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- ALOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of Micro/Mesoporous Materials by Physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Nagarkar, P.V.; Searson, P.C.; Gealy III, F.D. Effect of surface treatment on SrTiO3: An x-ray photoelectron spectroscopic study. J. Appl. Phys. 1991, 69, 459. [Google Scholar] [CrossRef]
- Shibagaki, S.; Fukushima, K. XPS analysis on Nb–SrTiO3 thin films deposited with pulsed laser ablation technique. J. Eur. Ceram. Soc. 1999, 19, 1423–1426. [Google Scholar] [CrossRef]
- Lefebvre, J.; Galli, F.; Bianchi, C.L.; Patience, G.-S.; Boffito, D.C. Experimental Methods in Chemical Engineering: X-Ray Photoelectron Spectroscopy—XPS. Can. J. Chem. Eng. 2019, 97, 2588–2593. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Liu, N.; Yokosawa, T.; Osvet, A.; Miehlich, M.E.; Meyer, K.; Spiecker, E.; Schmuki, P. Intrinsically Activated SrTiO3: Photocatalytic H2 Evolution from Neutral Aqueous Methanol Solution in the Absence of Any Noble Metal Cocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 29532–29542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miot, C.; Husson, E.; Proust, C.; Erre, R.; Coutures, J.P. Residual carbon evolution in BaTiO3 ceramics studied by XPS after ion etching. J. Eur. Ceram. Soc. 1998, 18, 339–343. [Google Scholar] [CrossRef]
- Liu, G.; Sun, C.; Yang, H.G.; Smith, S.C.; Wang, L.; Lu, G.Q.; Cheng, H.M. Nanosized anatase TiO2 single crystals for enhanced photocatalytic activity. Chem. Commun. 2010, 46, 755–757. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Alvarez-Docio, C.M.; del Campo, A.; Fernandez, J.F. Enhancement of UV absorption behavior inZnO–TiO2 composites. Bol. Soc. Esp. Ceram. Vidr. 2016, 55, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.-A.; Xu, J.-G.; Xiang, S.; Chen, L.-X.; Tan, Z.-G. Preparation of SrTiO3 nanoparticles by the combination of solid phase grinding and low temperature calcining. Mater. Lett. 2011, 65, 873–875. [Google Scholar] [CrossRef]
- Jia, A.; Liang, X.; Su, Z.; Zhu, T.; Liu, S. Synthesis and the effect of calcination tempera-ture on the physical-chemical properties and photocatalytic activities of Ni, La codoped SrTiO3. J. Hazard. Mater. 2010, 178, 233–242. [Google Scholar] [CrossRef]
- Yu, P.Y.; Cardona, M. Fundamentals of Semiconductors: Physics and Materials Properties, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. Factors affecting diclofenac decomposition in water by UV-A/TiO2 photocatalysis. Chem. Eng. J. 2010, 161, 53–59. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, S.; Kim, B.; Lee, S.; Lee, J.; Sim, S.; Gu, M.; Yi, J.; Lee, J. Toxic effects of titanium dioxide nanoparticles on microbial activity and metabolic flux. Biotechnol. Bioprocess Eng. 2012, 17, 276–282. [Google Scholar] [CrossRef]
- Oravisjärvi, K.; Pietikäinen, M.; Ruuskanen, J.; Rautio, A.; Voutilainen, A.; Keiski, R.L. Effects of physical activity on the deposition of traffic-related particles into the human lungs in silico. Sci. Total Environ. 2011, 409, 4511–4518. [Google Scholar] [CrossRef]
- Stucchi, M.; Alijani, S.; Manzoli, M.; Villa, A.; Lahti, R.; Galloni, M.G.; Lassi, U.; Prati, L. A Pt-Mo hybrid catalyst for furfural transformation. Catal. Today 2020, 327, 122–131. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Falletta, E.; Pifferi, V.; Falciola, L.; Cappelletti, G.; Ardizzone, S. Emerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium. Photochem. Photobiol. Sci. 2017, 16, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Species | Concentration (ppm) |
|---|---|
| Ca2+ | 44.5 |
| Na+ | 45.7 |
| Mg2+ | 9.3 |
| Cl− | 78.7 |
| SO42− | 36.5 |
| HCO3− | 121.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galloni, M.G.; Cerrato, G.; Giordana, A.; Falletta, E.; Bianchi, C.L. Sustainable Solar Light Photodegradation of Diclofenac by Nano- and Micro-Sized SrTiO3. Catalysts 2022, 12, 804. https://doi.org/10.3390/catal12080804
Galloni MG, Cerrato G, Giordana A, Falletta E, Bianchi CL. Sustainable Solar Light Photodegradation of Diclofenac by Nano- and Micro-Sized SrTiO3. Catalysts. 2022; 12(8):804. https://doi.org/10.3390/catal12080804
Chicago/Turabian StyleGalloni, Melissa G., Giuseppina Cerrato, Alessia Giordana, Ermelinda Falletta, and Claudia L. Bianchi. 2022. "Sustainable Solar Light Photodegradation of Diclofenac by Nano- and Micro-Sized SrTiO3" Catalysts 12, no. 8: 804. https://doi.org/10.3390/catal12080804
APA StyleGalloni, M. G., Cerrato, G., Giordana, A., Falletta, E., & Bianchi, C. L. (2022). Sustainable Solar Light Photodegradation of Diclofenac by Nano- and Micro-Sized SrTiO3. Catalysts, 12(8), 804. https://doi.org/10.3390/catal12080804