Vanadium Nitride Supported on N-Doped Carbon as High-Performance ORR Catalysts for Zn–Air Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization of VN/NC/C-x
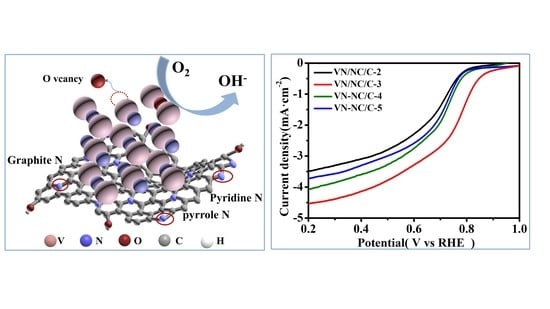
2.2. ORR Activity and Durability
2.3. Zn–Air Battery Performance
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of VN/NC/C-x
3.3. Electrochemical Measurements
3.4. Zn–Air Battery
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinde, S.S.; Lee, C.H.; Jung, J.-Y.; Wagh, N.K.; Kim, S.-H.; Kim, D.-H.; Lin, C.; Lee, S.U.; Lee, J.-H. Unveiling dual-linkage 3D hexaiminobenzene metal-organic frameworks towards long-lasting advanced reversible Zn-air batteries. Energy Environ. Sci. 2019, 12, 727–738. [Google Scholar] [CrossRef]
- Zhang, B.; Qiu, C.; Wang, S.; Gao, H.; Yu, K.; Zhang, Z.; Ling, X.; Ou, W.; Su, C. Electrocatalytic water-splitting for the controllable and sustainable synthesis of deuterated chemicals. Sci. Bull. 2021, 66, 562–569. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Xia, Y.; Ma, L.; Nie, C.; Roth, C.; Thomas, A.; Haag, R. Atomic Fe-Nx Coupled Open-Mesoporous Carbon Nanofibers for Efficient and Bioadaptable Oxygen Electrode in Mg-Air Batteries. Adv. Mater. 2018, 30, 1802669. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, M.; Zhou, W.; Duan, J.; Jin, W. Ultrathin 2D catalysts with N-coordinated single Co atom outside Co cluster for highly efficient Zn-air battery. Chem. Eng. J. 2021, 421, 129719. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Li, P.; Wang, L.; Zhang, H.; Liu, H.; Liu, J.; Wang, Y.; Tian, W.; Wang, X.; et al. Fe-N-doped porous carbon from petroleum asphalt for highly efficient oxygen reduction reaction. Carbon 2018, 126, 1–8. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhao, C.X.; Chen, S.; Liu, J.N.; Chen, X.; Song, L.; Zhang, Q. Framework-Porphyrin-Derived Single-Atom Bifunctional Oxygen Electrocatalysts and their Applications in Zn-Air Batteries. Adv. Mater. 2019, 31, 1900592. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, Z.; Li, M.; Yu, Z.; Zhang, M.; Gong, M.; Tang, Y.; Qiu, X. Coupling isolated Ni single atoms with sub-10 nm Pd nanocrystals embedded in porous carbon frameworks to boost oxygen electrocatalysis for Zn-air batteries. J. Mater. Chem. A 2022, 10, 6086–6095. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J.M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390. [Google Scholar] [CrossRef]
- Peng, X.; Pi, C.; Zhang, X.; Li, S.; Huo, K.; Chu, P.K. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain. Energy Fuels 2019, 3, 366–381. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, B. Rose-like, ruthenium-modified cobalt nitride nanoflowers grown in situ on an MXene matrix for efficient and stable water electrolysis. J. Mater. Chem. A 2021, 9, 20758–20765. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Ding, K.; Huang, C.; Li, Q.; Chu, P.K.; Huo, K. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
- Bi, W.; Hu, Z.; Li, X.; Wu, C.; Wu, J.; Wu, Y.; Xie, Y. Metallic mesocrystal nanosheets of vanadium nitride for high-performance all-solid-state pseudocapacitors. Nano Res. 2014, 8, 193–200. [Google Scholar] [CrossRef]
- Qi, W.; Meng, X.; Adimi, S.; Guo, H.; Thomas, T.; Li, F.; Jiang, H.; Liu, S.; Yang, M. A size tunable bimetallic nickel-zinc nitride as a multi-functional co-catalyst on nitrogen doped titania boosts solar energy conversion. Dalton Trans. 2020, 49, 4887–4895. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhou, T.; Ge, J.; Wu, C. Recent Advances on the Modulation of Electrocatalysts Based on Transition Metal Nitrides for the Rechargeable Zn-Air Battery. ACS Mater. Lett. 2020, 2, 1423–1434. [Google Scholar] [CrossRef]
- Tareen, A.K.; Priyanga, G.S.; Khan, K.; Pervaiz, E.; Thomas, T.; Yang, M. Nickel-Based Transition Metal Nitride Electrocatalysts for the Oxygen Evolution Reaction. ChemSusChem 2019, 12, 3941–3954. [Google Scholar] [CrossRef]
- Mosavati, N.; Salley, S.O.; Ng, K.Y.S. Characterization and electrochemical activities of nanostructured transition metal nitrides as cathode materials for lithium sulfur batteries. J. Power Sources 2017, 340, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Tian, X.; Luo, J.; Zeng, J.; Li, Y.; Song, H.; Liao, S. A Co-doped porous niobium nitride nanogrid as an effective oxygen reduction catalyst. J. Mater. Chem. A 2017, 5, 14278–14285. [Google Scholar] [CrossRef]
- Liu, B.; Huo, L.; Zhang, G.; Zhang, J. Ternary Hollow Mesoporous TiN/N-Graphene/Pt Hybrid Results in Enhanced Electrocatalytic Performance for Methanol Oxidation and Oxygen Reduction Reaction. Electrochim. Acta 2016, 213, 771–782. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Q.; Li, J.; Du, X.; Liu, G.; Wang, Y.; Wu, L.; Chen, Z. Ordered macroporous design of sacrificial Co/VN nano-heterojunction as bifunctional oxygen electrocatalyst for rechargeable zinc-air batteries. Chem. Eng. J. 2022, 433, 117893. [Google Scholar] [CrossRef]
- Xu, L.; Deng, D.; Tian, Y.; Li, H.; Qian, J.; Wu, J.; Li, H. Dual-active-sites design of CoNx anchored on zinc-coordinated nitrogen-codoped porous carbon with efficient oxygen catalysis for high-stable rechargeable zinc-air batteries. Chem. Eng. J. 2021, 408, 127321. [Google Scholar] [CrossRef]
- Luo, J.; Tian, X.; Zeng, J.; Li, Y.; Song, H.; Liao, S. Limitations and Improvement Strategies for Early-Transition-Metal Nitrides as Competitive Catalysts toward the Oxygen Reduction Reaction. ACS Catal. 2016, 6, 6165–6174. [Google Scholar] [CrossRef]
- Zhang, N.; Cao, L.; Feng, L.; Huang, J.; Kajiyoshi, K.; Li, C.; Liu, Q.; Yang, D.; He, J. Co,N-Codoped porous vanadium nitride nanoplates as superior bifunctional electrocatalysts for hydrogen evolution and oxygen reduction reactions. Nanoscale 2019, 11, 11542–11549. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Lv, F.; Fan, Q.; Zhao, Y.Q.; Zhang, Q.; Wang, W.; Cheng, F.; Xi, P.; Guo, S. NiO/CoN Porous Nanowires as Efficient Bifunctional Catalysts for Zn-Air Batteries. ACS Nano 2017, 11, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-R.; Xue, H.; Lv, L.; Sun, J.; Guo, N.; Song, T.; Dong, H.; Zhang, J.; Wang, Q. Unraveling the synergistic effect of defects and interfacial electronic structure modulation of pealike CoFe@Fe3N to achieve superior oxygen reduction performance. Appl. Catal. B Environ. 2021, 295, 120314. [Google Scholar] [CrossRef]
- Tang, H.; Luo, J.; Yu, J.; Zhao, W.; Song, H.; Liao, S. Nanoconfined Nitrogen-Doped Carbon-Coated Hierarchical TiCoN Composites with Enhanced ORR Performance. ChemElectroChem 2018, 5, 2041–2049. [Google Scholar] [CrossRef]
- Tang, H.; Luo, J.; Tian, X.L.; Dong, Y.; Li, J.; Liu, M.; Liu, L.; Song, H.; Liao, S. Template-Free Preparation of 3D Porous Co-Doped VN Nanosheet-Assembled Microflowers with Enhanced Oxygen Reduction Activity. ACS Appl. Mater. Interfaces 2018, 10, 11604–11612. [Google Scholar] [CrossRef]
- Sarkar, B.; Parui, A.; Singh, A.K.; Nanda, K.K. Mechanistic study on nitrogen-doped graphitic carbon-reinforced chromium nitride as a durable electrocatalyst for oxygen reduction. J. Mater. Chem. A 2021, 9, 16575–16584. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, Y.; Zhang, R.; Zhao, D. Synthesis of Self-Supported Ordered Mesoporous Cobalt and Chromium Nitrides. Adv. Funct. Mater. 2008, 18, 2436–2443. [Google Scholar] [CrossRef]
- Tsang, M.Y.; Pridmore, N.E.; Gillie, L.J.; Chou, Y.H.; Brydson, R.; Douthwaite, R.E. Enhanced photocatalytic hydrogen generation using polymorphic macroporous TaON. Adv. Mater. 2012, 24, 3406–3409. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhuang, M.; Ou, X.; Dou, Y.; Zhang, L.; Zhang, Q.; Wu, R.; Ding, Y.; Shao, M.; Luo, Z. Polymer-Embedded Fabrication of Co2P Nanoparticles Encapsulated in N, P-Doped Graphene for Hydrogen Generation. Nano Lett. 2016, 16, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Luo, J.; Nan, H.; Zou, H.; Chen, R.; Shu, T.; Li, X.; Li, Y.; Song, H.; Liao, S.; et al. Transition Metal Nitride Coated with Atomic Layers of Pt as a Low-Cost, Highly Stable Electrocatalyst for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 1575–1583. [Google Scholar] [CrossRef]
- Kelly, S.; Pollak, F.H.; Tomkiewicz, M. Raman Spectroscopy as a Morphological Probe for TiO2 Aerogels. J. Phys. Chem. B 1997, 101, 2730–2734. [Google Scholar] [CrossRef]
- Urry, D.W. Physical Chemistry of Biological Free Energy Transduction As Demonstrated by Elastic Protein-Based Polymers. J. Phys. Chem. B 1997, 101, 11007–11028. [Google Scholar] [CrossRef]
- Liu, X.; Yin, Q.; Dai, C.; Li, G.; Lian, J.; Zhao, Y.; Yang, S.; Li, H. Amorphous Bimetallic Phosphate-Carbon Precatalyst with Deep Self-Reconstruction toward Efficient Oxygen Evolution Reaction and Zn-Air Batteries. ACS Sustain. Chem. Eng. 2021, 9, 5345–5355. [Google Scholar] [CrossRef]
- Niu, H.-J.; Wang, A.-J.; Zhang, L.; Feng, J.-J. Bioinspired One-Step Pyrolysis Fabrication of 3D Porous Co, N, P-doped Carbon Nanosheets with Enriched CoNx Active Sites as High-Performance Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Battery. ACS Appl. Energy Mater. 2020, 3, 2781–2790. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Q.; Ni, Z.; Luo, K.; Liu, Y.; Yuan, D. CoNi Nanoalloys @ N-Doped Graphene Encapsulated in N-Doped Carbon Nanotubes for Rechargeable Zn-Air Batteries. ACS Sustain. Chem. Eng. 2021, 9, 13491–13500. [Google Scholar] [CrossRef]
- Zhang, K.; Mai, W.; Li, J.; Li, G.; Tian, L.; Hu, W. Bimetallic Co3.2Fe0.8N-Nitrogen-Carbon Nanocomposites for Simultaneous Electrocatalytic Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. ACS Appl. Nano Mater. 2019, 2, 5931–5941. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Song, R.; Yang, S.; Song, H. Hybrid 2D-0D Graphene-VN Quantum Dots for Superior Lithium and Sodium Storage. Adv. Energy Mater. 2016, 6, 1502067. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Z.; Wu, J. Vanadium Nitride@nitrogen-Doped Graphene as Zinc Ion Battery Cathode with High Rate Capability and Long Cycle Stability. Ind. Eng. Chem. Res. 2022, 61, 2955–2962. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; He, D.; Cao, L.; Li, G.; Guo, P.; Huang, J. Heterostructured VN/Mo2C Nanoparticles as Highly Efficient pH-Universal Electrocatalysts toward the Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2021, 9, 15202–15211. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, W.; Zhang, N.; Du, Z.; Zhong, C.; Yan, W.; Ju, H.; Chu, W.; Jiang, H.; Wu, C.; et al. Ultrathin Cobalt Oxide Layers as Electrocatalysts for High-Performance Flexible Zn-Air Batteries. Adv. Mater. 2019, 31, 1807468. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, H.; Xiong, P.; Li, G.; Qiu, T.; Gong, W.B.; Zhao, F.; Li, C.; Li, Q.; Wang, G.; et al. Molecularly Thin Nitride Sheets Stabilized by Titanium Carbide as Efficient Bifunctional Electrocatalysts for Fiber-Shaped Rechargeable Zinc-Air Batteries. Nano Lett. 2020, 20, 2892–2898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, P.; Sun, F.; Zhang, G.; Liu, X.; Wang, L. The cooperation of Fe3C nanoparticles with isolated single iron atoms to boost the oxygen reduction reaction for Zn-air batteries. J. Mater. Chem. A 2021, 9, 6831–6840. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Luo, Y.; Chen, Y.; Li, Z.; Shi, J.; Wang, G.; Wang, R. Synergistic coupling of Co4N/VN confined in N-doped carbon derived from zeolitic imidazolate frameworks for oxygen reduction reaction. Carbon 2020, 159, 16–24. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huo, L.; Si, R.; Liu, J.; Zhang, J. A General Method for Constructing Two-Dimensional Layered Mesoporous Mono- and Binary-Transition-Metal Nitride/Graphene as an Ultra-Efficient Support to Enhance Its Catalytic Activity and Durability for Electrocatalytic Application. ACS Appl. Mater. Interfaces 2016, 8, 18770–18787. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Min, Y.; Ma, L.-L.; Lu, J.-Y.; Li, H.-C.; Liu, W.-J.; Chen, J.-J.; Yu, H.-Q. Iron-nitrogen doped carbon with exclusive presence of FexN active sites as an efficient ORR electrocatalyst for Zn-air battery. Appl. Catal. B Environ. 2020, 268, 118405. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Yan, A.; Wan, H.; Chen, G.; Pan, J.; Zhang, N.; Qiu, T.; Ma, R.; Qiu, G. Hybrid Nanostructures of Bimetallic NiCo Nitride/N-Doped Reduced Graphene Oxide as Efficient Bifunctional Electrocatalysts for Rechargeable Zn-Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 19612–19620. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Liu, J.; Gao, C.; Jiang, L.; Lin, Y.; Meng, A. 3D Honeycomb Nanostructure Comprised of Mesoporous N-Doped Carbon Nanosheets Encapsulating Isolated Cobalt and Vanadium Nitride Nanoparticles as a Highly Efficient Electrocatalyst for the Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2020, 8, 3291–3301. [Google Scholar] [CrossRef]
- Song, J.; Yu, D.; Wu, X.; Xie, D.; Sun, Y.; Vishniakov, P.; Hu, F.; Li, L.; Li, C.; Maximov, M.Y.; et al. Interfacial coupling porous cobalt nitride nanosheets array with N-doped carbon as robust trifunctional electrocatalysts for water splitting and Zn-air battery. Chem. Eng. J. 2022, 437, 133509. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, H.; Li, Y.; Liang, D.; Hu, Y.; Li, C. In-situ enriching active sites on co-doped Fe-Co4N@N-C nanosheet array as air cathode for flexible rechargeable Zn-air batteries. Appl. Catal. B Environ. 2019, 256, 117893. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Han, L.; Zheng, P.; Peng, X.; Xian, X.; Liu, J.; Zeng, X.; Dong, P.; Xiao, J.; Zhang, Y. Vanadium Nitride Supported on N-Doped Carbon as High-Performance ORR Catalysts for Zn–Air Batteries. Catalysts 2022, 12, 877. https://doi.org/10.3390/catal12080877
Fu Y, Han L, Zheng P, Peng X, Xian X, Liu J, Zeng X, Dong P, Xiao J, Zhang Y. Vanadium Nitride Supported on N-Doped Carbon as High-Performance ORR Catalysts for Zn–Air Batteries. Catalysts. 2022; 12(8):877. https://doi.org/10.3390/catal12080877
Chicago/Turabian StyleFu, Yidan, Lina Han, Pengfei Zheng, Xianhui Peng, Xianglan Xian, Jinglin Liu, Xiaoyuan Zeng, Peng Dong, Jie Xiao, and Yingjie Zhang. 2022. "Vanadium Nitride Supported on N-Doped Carbon as High-Performance ORR Catalysts for Zn–Air Batteries" Catalysts 12, no. 8: 877. https://doi.org/10.3390/catal12080877
APA StyleFu, Y., Han, L., Zheng, P., Peng, X., Xian, X., Liu, J., Zeng, X., Dong, P., Xiao, J., & Zhang, Y. (2022). Vanadium Nitride Supported on N-Doped Carbon as High-Performance ORR Catalysts for Zn–Air Batteries. Catalysts, 12(8), 877. https://doi.org/10.3390/catal12080877