The CREC Fluidized Riser Simulator a Unique Tool for Catalytic Process Development
Abstract
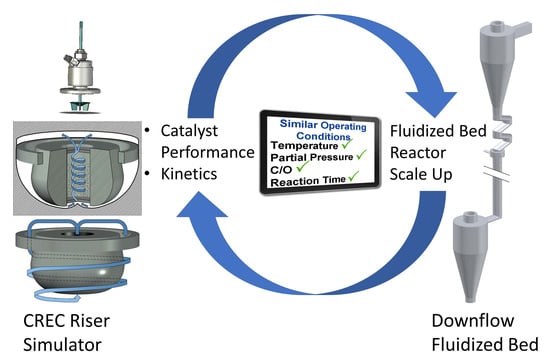
:1. Introduction
2. The 1992-CREC Riser Simulator Model and Its Auxiliary Equipment
3. Conversion and Selectivity in the 1992-CREC Riser Simulator
- To determine the TIPB Conversion parameter, the following equation can be used:with = being the chemical species change (excluding coke), β = /Wo representing the coke yield, Wi and WT standing for the “i” product and feedstock weights (excluding coke), respectively, and Ai and AT denoting the FID-GC areas for the “i” product and all FID-GC chemical species detected, respectively.
- 2.
- To calculate the product , representing the “i” product or lump of products over the feedstock weight, the following equation can be used:with being the yield of “i” chemical species (excluding coke).
- 3.
- To establish the , Equations (1) and (2) can be combined as follows:
4. Validation of Collected Experimental Data Using Carbon and Argon Balances
5. Kinetic Modeling in the 1992-CREC Riser Simulator
6. Recent Advances with the Improved 2019-CREC Riser Simulator
6.1. Improved Flow Patterns with Basket Frustoconical Shape
6.2. High Performance Filter Condenser (HPFC) and Canister Filter Condenser (CFC)
6.3. MIR (Medium InfraRed) Measurements in the 1992-CREC Riser Simulator
7. CREC Riser Simulator Applications
8. Conclusions and Future Perspectives
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Notation | |
| FID-GC area for “i” chemical species | |
| Adjusted area using Equation (A14) | |
| Refer to Equation (A16) | |
| Adjusted | |
| 1, 3, 5 TIP area used as a reference. | |
| concentration of “i” species with I = A, B, C, D, E, F (Kmole/cm3) | |
| Condition 1 | At injection condition |
| Condition 2 | Prior to evacuation |
| Condition 3 | After evacuation |
| Conversion | |
| Energy of activation for j reaction step (kJ/Kmole) | |
| Hydrocarbon Balance Closure at Condition 2, percentual basis (-) | |
| Hc2% | Hydrocarbon Balance Closure at Condition 3, percentual basis (-) |
| Inert balance closure at Condition 3, percentual basis (-) | |
| frequency factor for 1, 2, 3, 4, 5, 6, 7 reaction steps (1/s) | |
| apparent frequency factor for 1,2, 3 reaction step (1/s) | |
| Molecular Weight of inert (kg/Kmole). | |
| Hydrocarbon average molecular weight (kg/Kmole). | |
| Molecular weight of hydrocarbons at injection: Condition 1 (kg/Kmole) | |
| Molecular weight of hydrocarbons prior to evacuation: Condition 2 (kg/Kmole) | |
| Molecular weight in the reactor and in vacuum box after evacuation: Condition 3 (kg/Kmole) | |
| MWCHn,TIPB | Molecular weight ofthe CHn unit for TIPB (kg/Kmole) |
| MWCHn,VGO | Molecular weight of the CHn unit for VGO (kg/Kmole) |
| Molecular weight of vacuum gas oil | |
| Moles of hydrocarbons at injection condition: Condition 1 (Kmole) | |
| Moles of hydrocarbons in reactor prior to evacuation: Condition 2 (Kmole) | |
| Moles of hydrocarbons after evacuation: Condition 3 (Kmole) | |
| Moles of hydrocarbons in reactor at injection: Condition 1 (Kmole) | |
| Moles of hydrocarbons in reactor after evacuation: Condition 3 (Kmole) | |
| Moles of hydrocarbons in vacuum box after evacuation: Condition 3 (Kmole) | |
| Combined moles of inert in the reactor and vacuum box after evacuation: Condition 3 (Kmole) | |
| Moles of inert in the reactor at injection condition: Condition 1 (Kmole) | |
| Moles of inert in the vacuum box at injection condition: Condition 1 (Kmole) | |
| Moles of inert in reactor prior to evacuation (Kmole). | |
| Moles of inert in vacuum box after evacuation (Kmole) | |
| Equilibrium total pressure in reactor ad vacuum box, after evacuation: Condition 3 (atm) | |
| Total pressure at injection (atm) | |
| Inert gas total pressure in reactor, prior to hydrocarbon injection: Condition 1 (atm) | |
| Inert gas total pressure in vacuum box prior to evacuation: Condition 1 (atm) | |
| Inert gas total pressure in vacuum box after evacuation: Condition 3 (atm) | |
| Total pressure in reactor after hydrocarbon injection (atm): Condition 1 (atm) | |
| Total pressure in the reactor prior to evacuation: Condition 2 (atm) | |
| Coke concentration (gcoke/gcatalyst) | |
| Reaction rate for j step (Kmole/kgcat·s) | |
| Universal gas constant (atm·cm3/mole K) | |
| Yield/Conversion (-) | |
| Reaction time (s) | |
| Temperature in the reactor (K) | |
| Temperature in the Vacuum Box (K) | |
| Volume reactor (cm3) | |
| Volume vacuum box (cm3) | |
| Weight of catalyst (kg) | |
| Mass of coke formed (kg) | |
| Mass of hydrocarbons in reactor at time zero: Condition 1 (kg) | |
| Mass of hydrocarbons in reactor at “t” time prior to evacuation: Condition 2 (kg) | |
| Mass of hydrocarbons in reactor at “t” time prior to evacuation, including coke (kg) | |
| Mass of hydrocarbons in reactor and vacuum box after evacuation: Condition 3 (kg) | |
| Mass of hydrocarbons in reactor and vacuum box after evacuation, including coke (kg) | |
| Mass of i species (kg) | |
| Mass of inert gas in reactor at injection: Condition 1 (kg) | |
| Mass of inert gas in reactor at “t” total reaction time: Condition 2 (kg). | |
| Mass of inert gas remaining in the reactor after evacuation: Condition 3 (kg) | |
| Total mass of inert gas in reactor and vacuum box after evacuation: Condition 3 (kg) | |
| Weight of VGO sample injected (kg) | |
| Weight of pure 1, 3, 5 TIP analyzed and used as a reference (kg) | |
| Chemical species weight fractions as | |
| Greek Symbols | |
| (-) | |
| deactivation constant (kgcat/kgcoke) | |
| β | |
| FID-GC calibration factor for “i” species | |
| FID-GC calibration factor for the entire slate of products and reactants | |
| Catalytic deactivation function (-) | |
| γ | Methane area peak from 6 PVb loop over average several methane peaks from 6 PVb loop |
| Ratio of molecular weight of CHn unit in TIPB over molecular weight of CHn in VGO | |
| frequency factor for the j step (-) | |
| Acronyms | |
| C/O | Catalyst/Oil Ratio |
| CPFD | Computerized Particle Fluid Dynamics |
| CREC | Chemical Reactor Engineering Centre |
| MAT | Micro Activity Test |
| MTBE | Methyl-Ter-Butyl-Ether |
| PODH | Propane Oxidative Dehydrogenation |
| TIPB | Tri-isopropyl-benzene |
| VGO | Vacuum gas oil |
| 4PV | Four port valve |
| 6 PV | Six port valve |
| 6 PVa | Six port valve in the auxiliary heat vacuum chamber |
| 6 PVb | Six port valve in the 2019-CREC Riser Simulator auxiliary system for methane calibrations |
Appendix A. Validation of Hydrocarbon Catalytic Cracking of TIPB Runs in CREC Riser Simulator via Mass Balances
- Mass balances of hydrocarbons and inert gas at hydrocarbon injection time or Condition 1:
- 2.
- Mass balances of hydrocarbons and inert gas at the “t”, total reaction time:
- ●
- Mass balances at “t” reaction time or Condition 2 prior to evacuation:
- ●
- Mass balances at t = teq” or Condition 3, following product evacuation from the reactor:
- 3.
- Inert and hydrocarbon balance closure:



Appendix B. Validation of VGO Cracking Runs in the CREC Riser Simulator via Mass Balances
- (a)
- Obtain the areas from FID-GC in the C1–C16 range.
- (b)
- Adjusted = with
- (c)
- Carbon-based product weight fractions as
- (d)
- Average product molecular weight (MWp) as
- (e)
- VGO conversion aswith Equation (A17) including a TIPB amount and GC area from separate blank calibration experiments.
- (f)
- Average molecular weight for all chemical species (reactant and products) in the vacuum box and reactor or Condition 3:
- (g)
- Total product amount, using Equations (A7) and (A8) from Appendix A.
- (h)
- Inert%, using Equations (A10) and (A11) from Appendix A.
- (i)
References
- De Lasa, H. Riser Simulator. U.S. Patent 5102628A, 7 April 1992. [Google Scholar]
- De Lasa, H. Reactor and Multifunctional Riser and Downer Simulator. U.S. Patent 10220363, 5 March 2019. [Google Scholar]
- Islam, M.A.; Krol, S.; de Lasa, H. Slip Velocity in Downer Reactors: The Drag Coefficient and the Influence of Operational Variables. Ind. Eng. Chem. Res. 2010, 49, 6735. [Google Scholar] [CrossRef]
- Islam, M.; Krol, S.; de Lasa, H. The CREC-GS-Optiprobes and Its Focal Region. Gas-Solid Flow Measurements in Down Flow Reactors. Chem. Eng. Sci. 2011, 66, 1671. [Google Scholar] [CrossRef]
- Lanza, A.; Islam, M.A.; de Lasa, H. Particle Clusters and Drag Coefficients in Gas—Solid Downer Units. Chem. Eng. J. 2012, 200–202, 439–451. [Google Scholar] [CrossRef]
- Lanza, A.; de Lasa, H. CPFD Modeling and Experimental Validation of Gas-Solid Flow in a Down-Flow Reactor. Comput. Chem. Eng. 2016, 90, 79–93. [Google Scholar] [CrossRef]
- Lanza, A.; Islam, M.; de Lasa, H. Particle Cluster Sizing in Downer Units. Applicable Methodology Across Downer Scale Units. Powder Technol. 2017, 316, 198–206. [Google Scholar] [CrossRef]
- Medina Pedraza, C.; de Lasa, H. Hybrid Particle Cluster CPFD Simulation in the Acceleration and Stabilized Sections of a Downflow Circulating Fluidized Bed. Ind. Eng. Chem. Res. 2020, 59, 20235–20336. [Google Scholar] [CrossRef]
- Medina Pedraza, C.; de Lasa, H. Cluster Acceleration and Stabilization in a Downflow Circulating Fluidized Bed Unit. Ind. Eng. Chem. Res. 2020, 59, 12360–12370. [Google Scholar] [CrossRef]
- Pekediz, A.; Kraemer, D.W.; Chabot, J.; de Lasa, H.I. Mixing Patterns in a Novel Riser Simulator. In Chemical Reactor Technology for Environmentally Safe Reactors and Products; de Lasa, H., Doğu, G., Ravella, A., Eds.; Springe: Dordrecht, The Netherlands, 1992; Volume 225, p. 133. [Google Scholar] [CrossRef]
- Ginsburg, J.; Pekediz, A.; de Lasa, H. The CREC Fluidized Riser Simulator. Characterization of Mixing Patterns. Int. J. Chem. React. Eng. 2003, 1, A50. [Google Scholar] [CrossRef]
- Ahmed, I.; Rostom, S.; Lanza, A.; de Lasa, H. Computational Fluid Dynamics Study of the CREC Riser Simulator: Mixing Patterns. Power Technol. 2017, 316, 641–649. [Google Scholar] [CrossRef]
- Quddus, M.R. A Novel Mixed Metallic Oxygen Carrier for Chemical Looping Combustion: Preparation, Characterization and Kinetic Modeling Combustion: Preparation, Characterization and Kinetic Modeling. Ph.D. Dissertation, The University of Western Ontario, London, ON, Canada, 2013. [Google Scholar]
- Medina Pedraza, C.; Alkhlel, A.; de Lasa, H. Kinetic Modelling of 1, 3, 5-Triisopropylbenzene Catalytic Cracking Using a CREC Riser Simulator Emulating FCC Operation: The C/O Ratio Effect. Can. J. Chem. Eng. 2022. Submitted. [Google Scholar]
- Alkhlel, A.; de Lasa, H. Catalytic Cracking of Hydrocarbons in a CREC Riser Simulator Using a Y Zeolite-Based Catalyst: Assessing the Catalyst/Oil Ratio Effect. Ind. Eng. Chem. Res. 2018, 57, 13627–13638. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. High Propylene Selectivity via Propane Oxidative Dehydrogenation Using a Novel Fluidizable Catalyst: Kinetic Modeling. Ind. Eng. Chem. Res. 2018, 57, 10251–10260. [Google Scholar] [CrossRef]
- Lopez Zamora, S.; Alkhlel, A.; de Lasa, H. Monitoring the Progress of Catalytic Cracking for Model Compounds in the Mid-Infrared (MIR) 3200–2800 cm−1 Range. Chem. Eng. Sci. 2018, 192, 788–802. [Google Scholar] [CrossRef]
- Lopez Zamora, S.; de Lasa, H. A Mid-Infrared Region (MIR) Lumped Group Contribution Based Method for Monitoring Light Gases and Gasolines in Fluid Catalytic Cracking. Chem. Eng. Sci. 2020, 212, 115324. [Google Scholar] [CrossRef]
- Al-Khataff, S.; de Lasa, H. Activity and Selectivity of FCC Catalysts in a Riser Simulator. The Role of Y-Zeolite Crystal Size. Ind. Eng. Chem. Res. 1999, 38, 1350–1356. [Google Scholar] [CrossRef]
- Al-Khattaf, S.; Atias, J.; Jarosch, K.; de Lasa, H. Diffusion and Catalytic Cracking of 1,3,5 Tri-Isopropyl-Benzene in FCC Catalysts. Chem. Eng. Sci. 2002, 57, 4909–4920. [Google Scholar] [CrossRef]
- Alkhlel, A.; de Lasa, H. Catalyst/Feedstock Ratio Effect on FCC Using Different Catalysts Samples. Catalysts 2019, 9, 542. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; Atias, J.; de Lasa, H. Kinetic Modeling of Catalytic Cracking of Gas Oil Feedstocks: Reaction and Diffusion Phenomena. Ind. Eng. Chem. Res 2006, 45, 1583–1593. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; Atias, J.; de Lasa, H. Heterogeneous Approach to the Catalytic Cracking of Vacuum Gas Oil. Ind. Eng. Chem. Res. 2008, 47, 7631–7641. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; de Lasa, H. Kinetic Modeling of Catalytic Conversion of Methylcyclohexane over USY Zeolites: Adsorption and Reaction Phenomena. AIChEJ. 2009, 65, 1538–1558. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; de Lasa, H. Modeling Thermal and Catalytic Conversion of Decalin under Industrial FCC Operating Conditions. Chem. Eng. Sci. 2010, 65, 626–644. [Google Scholar] [CrossRef]
- Al Sabawi, M.; de Lasa, H. Influence of Zeolite Crystallite Size on Methyl-Cyclohexane Catalytic Conversion Products. Fuel 2012, 96, 511–523. [Google Scholar] [CrossRef]
- Arandes, J.; Ereña, J.; Olazar, M.; Bilbao, J.; de la Puente, G. Valorization of Polyolefin/LCO Blend over HZSM-5 Zeolites. Int. J. Chem. React. Eng. 2002, 1, A7. [Google Scholar] [CrossRef]
- Arandes, J.; Abajo, I.; Bilbao, J.; Azkoiti, M.; de Lasa, H. Consistency of the Ten Lump Model for Cracking: Study in a Laboratory Reactor and Use for Simulation of an FCCU. Chem. Eng. Commun. 2003, 190, 254–284. [Google Scholar] [CrossRef]
- Arandes, J.; Azkoiti, M.; Torre, I.; Olazar, M.; Castaño, P. Effect of HZSM-5 Catalyst Addition on the Cracking of Polyolefin Pyrolysis Waxes under FCC Conditions. Chem. Eng. J. 2007, 132, 17–26. [Google Scholar] [CrossRef]
- Arandes, J.; Torre, I.; Bilbao, J.; de Lasa, H. Effect of Catalyst Properties on the Cracking of Polypropylene Pyrolysis Waxes under FCC Conditions. Catal. Today 2008, 133–135, 413–419. [Google Scholar] [CrossRef]
- Atias, J.A.; Tonetto, G.; de Lasa, H. Modeling Catalytic Cracking in a Novel Riser Simulator: Adsorption Parameters under Reaction Conditions. Int. J. Chem. React. Eng. 2002, 1, A50. [Google Scholar] [CrossRef]
- Atias, J.A.; de Lasa, H. Adsorption, Diffusion and Reaction Phenomena over FCC Catalysts in the CREC Riser Simulator. Ind. Eng. Chem. Res. 2004, 43, 4709–4720. [Google Scholar] [CrossRef]
- Atias, J.A.; de Lasa, H. Adsorption and Catalytic Reaction in FCC Catalysts using a Novel Fluidized CREC Riser Simulator. Chem. Eng. Sci 2004, 59, 5663–5669. [Google Scholar] [CrossRef]
- García, J.; Falco, M.; Sedran, U. Intracrystallite Mesoporosity over Y Zeolites. Processing of VGO and Resid-VGO Mixtures in FCC. Catal. Today 2017, 296, 247–253. [Google Scholar] [CrossRef]
- Gianetto, A.; Farag, H.; Blasetti, A.; de Lasa, H. FCC Catalyst for Reformulated Gasolines. Kinetic Modelling. Ind. Eng. Res. 1994, 33, 3053–3062. [Google Scholar] [CrossRef]
- Ibarra, A.; Veloso, A.; Bilbao, J.; Arandes, J.; Castaño, P. Dual Coke Deactivation Pathways during the Catalytic Cracking of Raw Bio-Oil and Vacuum Gasoil in FCC Conditions. Appl. Catal. B Environ. 2016, 182, 336–346. [Google Scholar] [CrossRef]
- Ibarra, A.; Hita, I.; Arandes, J.; Bilbao, J. Influence of the Composition of Raw Bio-Oils on Their Valorization in Fluid Catalytic Cracking Conditions. Energy Fuels 2019, 33, 7458–7465. [Google Scholar] [CrossRef]
- Ibarra, A.; Hita, I.; Arandes, J.; Bilbao, J. A Hybrid FCC/HZSM-5 Catalyst for the Catalytic Cracking of a VGO/Bio-Oil Blend in FCC Conditions. Catalysts 2020, 10, 1157. [Google Scholar] [CrossRef]
- Ibarra, A.; Palos, R.; Arandes, J.; Olazar, M.; Bilbao, J.; de Lasa, H. Synergy in the Co-Cracking under FCC Conditions of a Phenolic Compound in the Bio-Oil (different word was found)and a Model Compound for Vacuum Gasoil. Ind. Eng. Chem. Res. 2020, 59, 8145–8154. [Google Scholar] [CrossRef]
- Jiménez-García, G.; de Lasa, H.; Quintana, R.; Maya-Yescas, R. Catalyst Activity Decay Due to Pore Blockage during Catalytic Cracking of Hydrocarbons. Fuel 2013, 110, 89–98. [Google Scholar] [CrossRef]
- Jiménez-García, G.; de Lasa, H.; Maya Yescas, R. Simultaneous Estimation of Kinetics and Catalysts Activity during Cracking of 1,3,5-Tri-Isopropyl Benzene on FCC Catalyst. Catal. Today 2014, 220–222, 178–185. [Google Scholar] [CrossRef]
- Kraemer, D.; de Lasa, H. Catalytic Cracking of Hydrocarbons in a Riser Simulator. Chem. Eng. Sci. 1988, 27, 2002–2008. [Google Scholar] [CrossRef]
- Kraemer, D.; Sedran, U.; de Lasa, H. Catalytic Cracking Kinetics in a Novel Riser Simulator. Chem. Eng. Sci. 1990, 45, 2447–2452. [Google Scholar] [CrossRef]
- Martignoni, W.; de Lasa, H. Heterogeneous Reaction Model for FCC Riser Units. Chem. Eng. Sci 2001, 56, 605–612. [Google Scholar] [CrossRef]
- Ochoa, A.; Vicente, H.; Sierra, I.; Arandes, J.; Castaño, P. Implications of Feeding or Cofeeding Bio-Oil in the Fluid Catalytic Cracker (FCC) in terms of Regeneration Kinetics and Energy Balance. Energy 2020, 209, 118467. [Google Scholar] [CrossRef]
- Pruski, J.; Pekediz, A.; de Lasa, H. Catalytic Cracking of Hydrocarbons in a Novel Riser Simulator: Lump Adsorption Parameters under Reaction Conditions. Chem. Eng. Sci. 1996, 51, 1799–1806. [Google Scholar] [CrossRef]
- Torre, I.; Arandes, J.; Castaño, P.; Azkoiti, M.J.; Bilbao, J.; de Lasa, H. Catalytic Cracking of Plastic Pyrolysis Waxes with Vacuum Gasoil: Effect of HZSM-5 Zeolite in the FCC Catalyst. Int. J. Chem. React. Eng. 2006, 4, A31. [Google Scholar] [CrossRef]
- Hita, I.; Arandes, J.M.; Bilbao, J. Upgrading of Bio-oil via Fluid Catalytic Cracking. In Chemical Catalysts for Biomass Upgrading; Crocker, M., Santillan-Jimenez, E., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 61–96. [Google Scholar] [CrossRef]
- Palos, I.; Rodriguez, E.; Gutierrez, A.; Bilbao, J.; Arandes, J.M. Cracking of Plastics pyrolysis oil over FCC equilibrium catalysts to produce fuels: Kinetic modelling. Fuel 2022, 316, 123341. [Google Scholar] [CrossRef]
- Adnan, M.; Razzak, S.; Hossein, M.; de Lasa, H. Iron Oxide over Silica-Doped Alumina Catalyst for Catalytic Steam Reforming of Toluene as Surrogate Tar Biomass Species. Energy Fuels 2017, 31, 7471–7481. [Google Scholar] [CrossRef]
- Calzada, A.; Gonzalez, S.; Sanchez, A.; de Lasa, H.; Serrano, B. Ru-Promoted Ni/Al2O3 Fluidized Catalyst for Biomass Gasification. Catalysts 2020, 10, 316. [Google Scholar] [CrossRef]
- Calzada Hernandez, A.; Serrano, B.; de Lasa, H. Kinetic Model of Catalytic Steam Gasification of 2-Methoxy-4-Methylphenol Using 5% Ni–0.25% Ru/γAl2O3 in a CREC-Riser Simulator. Catalysts 2022, 12, 282. [Google Scholar] [CrossRef]
- de Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef]
- Kuhn-Bastos, A.; Torres, C.; Mazumder, A.; de Lasa, H. CO2 Biomass Fluidized Gasification: Thermodynamics and Reactivity Studies. Can. J. Chem. Eng. 2018, 96, 2176–2184. [Google Scholar] [CrossRef]
- Mazumder, A.; de Lasa, H. Fluidizable Ni/La2O3-γAl2O3 Catalyst for Steam Gasification of Cellulosic Biomass Surrogate. Appl. Catal. B Environ. 2014, 160–161, 67–79. [Google Scholar] [CrossRef]
- Mazumder, A.; de Lasa, H. Fluidizable La2O3 Promoted Ni/ γ-Al2O3 Catalyst for Steam Gasification of Biomass: Effect of Catalyst Preparation Conditions. Appl. Catal. B Environ. 2015, 168-169, 250–265. [Google Scholar] [CrossRef]
- Mazumder, A.; de Lasa, H. Steam Gasification of a Cellulosic Biomass Surrogate Using a Ni/La2O3-γAl2O3 Catalyst in a CREC Fluidized Riser Simulator. Kinetics and Model Validation. Fuel 2018, 216, 101–109. [Google Scholar] [CrossRef]
- Salaices, E.; Serrano, B.; de Lasa, H. Steam Gasification of a Cellulose Surrogate Over Fluidizable Ni/α-Alumina Catalyst: A Kinetics Model. AIChEJ 2012, 58, 1588–1599. [Google Scholar] [CrossRef]
- Torres, C.; Rostom, S.; de Lasa, H. An Eco-Friendly Fluidizable FexOy/CaO-γ-Al2O3 Catalyst for Tar Cracking during Biomass Gasification. Catalysts 2020, 10, 806. [Google Scholar] [CrossRef]
- Ahmed, I.; de Lasa, H. Biomass derived CO2 Capture Using Chemical Looping Combustion: Simulation of a Riser-Downer Scaled-Up Configuration. Ind. Eng. Chem. Res. 2020, 59, 6900–6913. [Google Scholar] [CrossRef]
- Ahmed, I.; de Lasa, H. Syngas Chemical Looping Combustion using a Highly Performing Fluidizable Oxygen Carrier. Catalysis Today 2020, 343, 63–71. [Google Scholar] [CrossRef]
- Ahmed, I.; de Lasa, H. 110th Anniversary: Kinetic Model for Syngas Chemical Looping Combustion Using a Nickel-Based Highly Performing Fluidizable Oxygen Carrier. Ind. Eng. Chem. Res. 2019, 58, 2801–2811. [Google Scholar] [CrossRef]
- Hossain, M.; de Lasa, H. Reduction and Oxidation Kinetics of Co-Ni/Al2O3 Oxygen Carrier Involved in a Chemical Looping Combustion Cycles. Chem. Eng. Sci 2009, 65, 98–106. [Google Scholar] [CrossRef]
- Hossain, M.; Lopez, D.; Herrera, J.; de Lasa, H. Nickel on Lanthanum-Modified γAl2O3 Oxygen Carrier for CLC: Reactivity and Stability. Catal. Today 2009, 143, 179–186. [Google Scholar] [CrossRef]
- Hossain, M.; Quddus, M.R.; de Lasa, H. Reduction Kinetics of Lanthanum Modified Ni/γ-Al2O3 Oxygen Carrier for CLC. Reduction Kinetics of La Modified NiO/La-γAl2O3 Oxygen Carrier for Chemical-Looping Combustion. (different title on internet). Ind. Eng. Chem. Res. 2010, 49, 11009–11017. [Google Scholar] [CrossRef]
- Quddus, M.; Hossain, M.; de Lasa, H. Ni Based Oxygen Carrier over γ-Al2O3 for Chemical Looping Combustion: Effect of Preparation Method on Metal Support Interaction. Catal. Today 2013, 210, 124–134. [Google Scholar] [CrossRef]
- Al-Bogami, S.; de Lasa, H. Kinetic Modeling of Benzothiophene Catalytic Conversion over a HZSM5 Based Catalyst. Ind. Eng. Chem. Res. 2013, 52, 17760–17772. [Google Scholar] [CrossRef]
- Al Bogami, S.; de Lasa, H. Catalytic Conversion of Benzothiophene Over a H-ZSM5 Based Catalyst. Fuel 2013, 108, 490–501. [Google Scholar] [CrossRef]
- Aponte, Y.; Djaouadi, D.; de Lasa, H. Sulfur Reduction Using a HIPZD Additive in a FCC Aromatic Gasoline Environment. Fuel 2014, 128, 71–87. [Google Scholar] [CrossRef]
- Aponte, Y.; Che-Galicia, G.; de Lasa, H. A Fluidizable Zn-Offretite for Selective Thiophenic Species Adsorption. Additive Performance under FCC Conditions. Fuel 2016, 186, 222–234. [Google Scholar] [CrossRef]
- Jaimes, L.; Ferreira, M.L.; de Lasa, H. Thiophene Conversion under Mild Conditions over a ZSM-5 Catalyst. Chem. Eng. Sci. 2009, 64, 2539–2561. [Google Scholar] [CrossRef]
- Jaimes, L.; de Lasa, H. Catalytic Conversion of Thiophene under Mild Conditions over A ZSM-5 Catalyst. A Kinetic Model. Ind. Eng. Chem. Res. 2009, 48, 7505–7516. [Google Scholar] [CrossRef]
- Jaimes, L.; Badillo, M.A.; de Lasa, H. FCC Gasoline Desulfurization Using a ZSM-5 Catalyst. Interactive Effects of Sulfur Containing Species and Gasoline Components. Fuel 2011, 90, 2016–2025. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Volpe, M.; Hossain, M.; de Lasa, H. VOx/c-Al2O3 Catalyst for Oxidative Dehydrogenation of Ethane to Ethylene: Desorption Kinetics and Catalytic Activity. Appl. Catal. A Gen. 2013, 450, 120–130. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Hossain, M.; de Lasa, H. Kinetic Modeling of Ethane Oxidative Dehydrogenation over VOx/Al2O3 Catalyst in a Fluidized-Bed Riser Simulator. Ind. Eng. Chem. Res. 2013, 52, 5235–5244. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; de Lasa, H. Propane Oxidative Dehydrogenation over a VOx/Al2O3 Catalyst: Characterization and Catalytic Performance. Fuel 2014, 128, 120–140. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Moreira, J.; de Lasa, H. Kinetic Modeling of Propane Oxidative Dehydrogenation over VOx/γ-Al2O3 Catalysts in the Chemical Reactor Engineering Center Riser Reactor Simulator. Ind. Eng. Chem. Res. 2014, 53, 15317–15332. [Google Scholar] [CrossRef]
- Ayandiran, A.; Bakare, I.; Binous, H.; Al-Ghamdi, S.; Razzak, S.; Hossain, M. Oxidative Dehydrogenation of Propane to Propylene over VOx/CaO–γ-Al2O3 Using Lattice Oxygen. Catal. Sci. Technol. 2016, 6, 5154. [Google Scholar] [CrossRef]
- Bakare, I.; Al-Ghamdi, S.; Razzak, S.; Hossain, M.; de Lasa, H. Fluidized bed ODH of Ethane to Ethylene over a VOx–MoOx/γ-Al2O3 Catalyst: Desorption Kinetics and Catalytic Activity. Chem. Eng. J. 2015, 278, 207–216. [Google Scholar] [CrossRef]
- Elbadawi, A.; Ba-Shammakh, M.S.; Al-Ghamdi, S.; Razzak, S.; Hossain, M.; de Lasa, H. Phenomenologically Based Kinetics of ODH of Ethane to Ethylene Using Lattice Oxygen of VOx/Al2O3-ZrO2 Catalyst. Chem. Eng. Res. Des. 2017, 117, 733–745. [Google Scholar] [CrossRef]
- Khan, M.; Al-Ghamdi, S.; Hossain, M.; de Lasa, H. Fluidized Bed Oxidative Dehydrogenation of Ethane to Ethylene over VOx/Ce-γAl2O3 Catalysts: Reduction Kinetics and Catalyst Activity. Mol. Catal. 2017, 443, 78–91. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Propane Oxidative Dehydrogenation Using Consecutive Feed Injections and Fluidizable VOx/γAl2O3 and VOx/ZrO2-γAl2O3 Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13109–13124. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Propane Oxidative Dehydrogenation on Vanadium-Based Catalysts under Oxygen-Free Atmospheres. Catalysts 2020, 10, 418. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Downer Fluidized Bed Reactor Modeling for Catalytic Propane Oxidative Dehydrogenation with High Propylene Selectivity. Chem. Eng. Process.-Process Intensification 2020, 137, 87–99. [Google Scholar] [CrossRef]
- El Solh, T.; Jarosch, K.; de Lasa, H. Fluidizable Catalyst for Methane Reforming. Appl. Catal. A Gen. 2001, 210, 315–324. [Google Scholar] [CrossRef]
- El Solh, T.; Jarosch, K.; de Lasa, H. Catalytic Dry Reforming of Methane in a CREC Riser Simulator: Kinetic Modeling and Model Discrimination. Ind. Eng. Chem. Res. 2003, 42, 2507–2515. [Google Scholar] [CrossRef]
- Jarosch, K.; de Lasa, H. Novel Riser Simulator for Methane Reforming Using High Temperature Membranes. Chem. Eng. Sci. 1999, 54, 1455–1460. [Google Scholar] [CrossRef]
- Jarosch, K.; de Lasa, H. Permeability, Selectivity and Testing of Hydrogen Diffusion Membranes Suitable for Use in Steam Reforming. Ind. Eng. Chem. Res. 2001, 40, 5391–5397. [Google Scholar] [CrossRef]
- Jarosch, K.; El Solh, T.; de Lasa, H. Modeling the Catalytic Steam Reforming of Methane: Discrimination between Kinetic Expressions Using Sequentially Designed Experiments. Chem. Eng. Sci. 2002, 57, 3439–3451. [Google Scholar] [CrossRef]
- De Lasa, H.; Fournier, P.; Prakash, A.; El Solh, T. MTBE Synthesis in a Novel Riser Simulator. Can. J. Chem. Eng. 1999, 77, 413–419. [Google Scholar] [CrossRef]












| Studies | Ref | Year | Approach | |
|---|---|---|---|---|
| 1 | Downflow reactors | [3] | 2010 | Fluid dynamic studies with CREC Optiprobes |
| 2 | [4] | 2011 | Fluid dynamic studies with CREC Optiprobes | |
| 3 | [5] | 2012 | CPFD Studies | |
| 5 | [7] | 2017 | Fluid dynamic studies with CREC Optiprobes | |
| 6 | [8] | 2020 | Fluid dynamic studies with CREC Optiprobes | |
| 7 | [9] | 2020 | CPFD Studies |
| Operating Conditions | CREC Riser Simulator | MAT (Micro Activity Test) Fixed Bed | MAT (Micro Activity Test)-Fluidized Bed |
|---|---|---|---|
| Temperature | Adequate | Adequate | Adequate |
| C/O | C/O is adequate. C/O is established on a weight ratio basis. | C/O is not acceptable. C/O is established on a cumulative basis. | C/O is not acceptable. C/O is established on a cumulative basis. |
| Reaction times | Reaction times for both gas phase and catalyst phase are the same. | Reaction times for the gas phase and the catalyst phase are significantly different. | Reaction times for the gas phase and the catalyst phase are significantly different. |
| Partial Pressure | Partial pressures are in the proper range. | Partial pressures are significantly lower than in the industrial scale unit. | Partial pressures are significantly lower than in the industrial scale unit. |
| Catalyst activity and coke | Catalyst activity and coke levels are uniform throughout the bed, at specific reaction time. | Catalyst activity and coke levels vary considerably throughout the bed, at a given catalyst time-on-stream. | Catalyst activity and coke levels are uniform throughout the bed at a given catalyst time-on-stream. |
| C/O = 5 | Step | Operating Regime | |||
|---|---|---|---|---|---|
| 1 | 0.13 ± 2.23% | 19,181 ± 2.5% | 0.04 | Diffusion-controlled | |
| 2 | 0.51 ± 4.24% | 31,687 ± 2.9% | 0.05 | Diffusion-controlled | |
| 3 | 1.93 ± 7.53% | 50,916 ± 3.2% | 0.32 | Diffusion- controlled |
| Parameters | Value | 95% CI | Correlation Matrix | |||||
|---|---|---|---|---|---|---|---|---|
| k10 | k20 | k30 | E1 | E2 | E3 | |||
| k10 a | 2.82 × 10–5 | ±1.15 × 10–6 | 1 | |||||
| k20 | 1.65 × 10–6 | ±1.02 × 10–7 | –0.84 | 1 | ||||
| k30 | 4.80 × 10–6 | ±2.29 × 10–6 | 0.83 | –0.94 | 1 | |||
| E1 b | 55.7 | ±7.58 | –0.21 | 0.04 | –0.20 | 1 | ||
| E2 | 33.3 | ±3.16 | –0.03 | 0.07 | 0.13 | –0.68 | 1 | |
| E3 | 98.5 | ±15.56 | 0.52 | –0.55 | 0.75 | –0.59 | 0.70 | 1 |
| m | 189 | |||||||
| DOF | 183 | |||||||
| N | Studies and Applications | Ref. | Year | Approach |
|---|---|---|---|---|
| 1 | Mixing and Tracers in the CREC Riser Simulator | [10] | 1992 | Mixing and tracer studies |
| 2 | [11] | 2003 | Mixing studies | |
| 3 | [12] | 2017 | Mixing studies | |
| 4 | Catalytic Cracking of Hydrocarbons | [19] | 1999 | FCC catalyst performance |
| 5 | [20] | 2002 | FCC catalysts: diffusion and kinetics | |
| 6 | [21] | 2019 | FCC catalyst performance: C/O ratio | |
| 7 | [22] | 2006 | VGO cracking kinetics | |
| 8 | [23] | 2008 | VGO conversion performance | |
| 9 | [24] | 2009 | VGO cracking kinetics | |
| 10 | [25] | 2010 | FCC catalyst performance | |
| 11 | [26] | 2012 | FCC performance with submicron zeolites | |
| 12 | [27] | 2002 | FCC polyolefins/LCO cracking | |
| 13 | [28] | 2003 | VGO FCC cracking kinetics | |
| 14 | [29] | 2007 | Polyolefin pyrolysis wax cracking at FCC conditions | |
| 15 | [30] | 2008 | FCC catalyst properties for polyolefin pyrolysis wax cracking | |
| 16 | [31] | 2002 | FCC and adsorption kinetics | |
| 17 | [32] | 2004 | FCC adsorption, diffusion, kinetics | |
| 18 | [33] | 2004 | FCC adsorption and kinetics | |
| 19 | [34] | 2017 | VGO FCC cracking and crystallites | |
| 20 | [35] | 1994 | FCC catalysts for reformulated gasoline | |
| 21 | [36] | 2016 | FCC catalyst deactivation by coke with VGO-Bio-oil | |
| 22 | [37] | 2019 | FCC catalyst performance with VGO-Bio-oil | |
| 23 | [38] | 2020 | FCC/HZSM-5 catalyst for catalytic cracking of VGO-Bio-oil | |
| 24 | [39] | 2020 | FCC co-cracking of biooil and VGO | |
| 25 | [40] | 2013 | FCC catalyst deactivation by coke | |
| 26 | [41] | 2014 | FCC cracking kinetics and catalyst activity | |
| 27 | [42] | 1990 | FCC cracking kinetics | |
| 28 | [43] | 1990 | FCC catalyst performance | |
| 29 | [44] | 2001 | FCC heterogeneous kinetics | |
| 30 | [45] | 2020 | FCC co-feeding bio-oil in an FCC unit | |
| 31 | [46] | 1996 | FCC lump kinetics for FCC | |
| 32 | [47] | 2006 | FCC cracking for plastic derived waxes | |
| 33 | [48] | 2020 | FCC for upgrading of bio-oil | |
| 34 | [49] | 2020 | FCC cracking of plastic pyrolysis oil | |
| 35 | Biomass Gasification and Conversion of Biomass Derived Tars | [50] | 2017 | Catalytic biomass derived tar conversion |
| 36 | [51] | 2020 | Ru-Ni-Al2O3 catalyst: performance | |
| 37 | [52] | 2022 | Ru-Ni-Al2O3 catalyst: kinetics | |
| 38 | [53] | 2011 | Kinetics and thermodynamics | |
| 39 | [54] | 2018 | Catalytic CO2 biomass gasification | |
| 40 | [55] | 2014 | Ni-La2O3-Al2O3 catalyst: performance | |
| 41 | [56] | 2015 | Ni-La2O3-Al2O3 catalyst: preparation | |
| 42 | [57] | 2018 | Ni-La2O3-Al2O3 catalyst: kinetics | |
| 43 | [58] | 2012 | Ni-Al2O3 catalyst performance | |
| 44 | [59] | 2020 | FexOy-CaO-Al2O3 catalyst performance | |
| 45 | Chemical Looping Combustion | [60] | 2020 | Biomass derived CO2 capture: CPFD simulation |
| 46 | [61] | 2020 | Highly performing oxygen carrier | |
| 47 | [62] | 2019 | Ni-Co-Al2O3 oxygen carrier: kinetics | |
| 48 | [63] | 2009 | Ni-Co-Al2O3 oxygen carrier: performance | |
| 49 | [64] | 2009 | Ni-La2O3-Al2O3 oxygen carrier: performance | |
| 50 | [65] | 2010 | Ni-La2O3-Al2O3: kinetics | |
| 51 | [66] | 2013 | Ni-Al2O3 oxygen carrier: preparation | |
| 52 | Catalytic Desulfurization of Gasoline | [67] | 2013 | Catalytic benzothiophene conversion: kinetics |
| 53 | [68] | 2013 | Catalytic benzothiophene conversion: catalyst performance | |
| 54 | [69] | 2014 | Sulfur reduction with HIPZD additive | |
| 55 | [70] | 2016 | Zn-offretite for thiophene adsorption | |
| 56 | [71] | 2009 | Thiophene conversion with ZSM5: performance. | |
| 57 | [72] | 2009 | Thiophene conversion with ZSM5: kinetics | |
| 58 | [73] | 2011 | Gasoline desulfurization with ZSM5 catalyst | |
| 59 | Paraffin Oxydehydrogenation | [74] | 2013 | V2O5-Al2O3 desorption and catalytic activity |
| 60 | [75] | 2013 | V2O5-Al2O3 kinetics: ethane ODH | |
| 61 | [76] | 2014 | V2O5-Al2O3 performance: propane ODH | |
| 62 | [77] | 2014 | V2O5-Al2O3 kinetics: propane ODH | |
| 63 | [78] | 2016 | VOx-CaOAl2O3 kinetics: propane ODH | |
| 64 | [79] | 2015 | VOx-MoOx-Al2O3 kinetics: propane ODH | |
| 65 | [80] | 2017 | VOx-MoOx-Al2O3 kinetics: propane ODH | |
| 66 | [81] | 2017 | VOx-ZrO2-Al2O3 kinetics: propane ODH | |
| 67 | [82] | 2017 | VOx-ZrO2-Al2O3: propane ODH | |
| 68 | [83] | 2017 | VOx catalysts: propane ODH | |
| 69 | [84] | 2020 | ODH catalysis with high propylene | |
| 70 | Catalytic Steam and Dry Reforming | [85] | 2001 | Catalytic steam methane reforming |
| 71 | [86] | 2003 | Catalytic dry reforming of methane | |
| [87] | 1999 | Catalytic steam methane reforming with membranes | ||
| 72 | [88] | 2001 | Methane steam reforming with membranes: Selectivity | |
| 73 | [89] | 2002 | Methane steam reforming with membranes: Selectivity: kinetics | |
| 74 | MTBE Synthesis | [90] | 1999 | MTBE Synthesis: catalyst performance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lasa, H. The CREC Fluidized Riser Simulator a Unique Tool for Catalytic Process Development. Catalysts 2022, 12, 888. https://doi.org/10.3390/catal12080888
de Lasa H. The CREC Fluidized Riser Simulator a Unique Tool for Catalytic Process Development. Catalysts. 2022; 12(8):888. https://doi.org/10.3390/catal12080888
Chicago/Turabian Stylede Lasa, Hugo. 2022. "The CREC Fluidized Riser Simulator a Unique Tool for Catalytic Process Development" Catalysts 12, no. 8: 888. https://doi.org/10.3390/catal12080888
APA Stylede Lasa, H. (2022). The CREC Fluidized Riser Simulator a Unique Tool for Catalytic Process Development. Catalysts, 12(8), 888. https://doi.org/10.3390/catal12080888