Integrated CO2 Capture and Hydrogenation to Produce Formate in Aqueous Amine Solutions Using Pd-Based Catalyst
Abstract
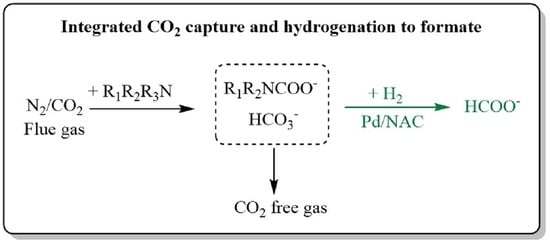
:1. Introduction
2. Results and Discussion
2.1. Integrated CO2 Capture and Hydrogenation to Produce Formate
2.2. Catalyst Characterization
2.3. Reaction Mechanism
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, M.; Ma, Y.; Zhou, Y.; Liu, C.; Qin, Y.; Fang, Y.; Guan, G.; Li, X.; Zhang, Z.; Wang, T. Influence of Transition Metal on the Hydrogen Evolution Reaction over Nano-Molybdenum-Carbide Catalyst. Catalysts 2018, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ren, B.; Ou, J.Z.; Xu, K.; Yang, C.; Li, Y.; Zhang, H. Engineering two-dimensional metal oxides and chalcogenides for enhanced electro- and photocatalysis. Sci. Bull. 2021, 66, 1228–1252. [Google Scholar] [CrossRef]
- Gao, R.; Deng, M.; Yan, Q.; Fang, Z.; Li, L.; Shen, H.; Chen, Z. Structural Variations of Metal Oxide-Based Electrocatalysts for Oxygen Evolution Reaction. Small Methods 2021, 5, 2100834. [Google Scholar] [CrossRef]
- Zeng, H.; Xing, B.; Cao, Y.; Xu, B.; Hou, L.; Guo, H.; Cheng, S.; Huang, G.; Zhang, C.; Sun, Q. Insight into the microstructural evolution of anthracite during carbonization-graphitization process from the perspective of materialization. Int. J. Min. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Li, L.; Conway, W.; Burns, R.; Maeder, M.; Puxty, G.; Clifford, S.; Yu, H. Investigation of metal ion additives on the suppression of ammonia loss and CO2 absorption kinetics of aqueous ammonia-based CO2 capture. Int. J. Greenh. Gas Control 2017, 56, 165–172. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Conway, W.; Puxty, G.; Burns, R.; Clifford, S.; Maeder, M.; Yu, H. The effect of piperazine (PZ) on CO2 absorption kinetics into aqueous ammonia solutions at 25.0 °C. Int. J. Greenhouse Gas. Control 2015, 36, 135–143. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Chen, Z.; Gao, R.; Yu, H.; Yuan, T.; Liu, Z.; Maeder, M. Heterogeneous catalysts for the hydrogenation of amine/alkali hydroxide solvent captured CO 2 to formate: A review. Greenh. Gases Sci. Technol. 2021, 11, 807–823. [Google Scholar] [CrossRef]
- Li, Y.-N.; He, L.-N.; Liu, A.-H.; Lang, X.-D.; Yang, Z.-Z.; Yu, B.; Luan, C.-R. In situ hydrogenation of captured CO2 to formate with polyethyleneimine and Rh/monophosphine system. Green Chem. 2013, 15, 2825–2829. [Google Scholar] [CrossRef]
- McNamara, N.D.; Hicks, J.C. CO2 Capture and Conversion with a Multifunctional Polyethyleneimine-Tethered Iminophosphine Iridium Catalyst/Adsorbent. ChemSusChem 2014, 10, 1114–1124. [Google Scholar] [CrossRef]
- Guan, C.; Pan, Y.; Ang, E.P.L.; Hu, J.; Yao, C.; Huang, M.H.; Li, H.; Lai, Z.; Huang, K.-W. Conversion of CO2 from air into formate using amines and phosphorus-nitrogen PN3P-Ru(ii) pincer complexes. Green Chem. 2018, 20, 4201–4205. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, J.; Yao, Y.; Sun, J.; Wang, Y.; Lin, H. Renewable energy storage via efficient reversible hydrogenation of piperidine captured CO2. Green Chem. 2018, 20, 4292–4298. [Google Scholar] [CrossRef]
- González, E.; Marchant, C.; Sepúlveda, C.; García, R.; Ghampson, I.; Escalona, N.; García-Fierro, J.L. Hydrogenation of sodium hydrogen carbonate in aqueous phase using metal/activated carbon catalysts. Appl. Catal. B Environ. 2018, 224, 368–375. [Google Scholar] [CrossRef]
- Stalder, C.J.; Chao, S.; Summers, D.P.; Wrighton, M.S. Supported palladium catalysts for the reduction of sodium bicarbonate to sodium formate in aqueous solution at room temperature and one atmosphere of hydrogen. J. Am. Chem. Soc. 1983, 105, 6318–6320. [Google Scholar] [CrossRef]
- Su, J.; Yang, L.; Lu, M.; Lin, H. Highly Efficient Hydrogen Storage System Based on Ammonium Bicarbonate/Formate Redox Equilibrium over Palladium Nanocatalysts. ChemSusChem 2015, 8, 813–816. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Lin, J.-D.; Liu, Y.-M.; Du, X.-L.; Wang, J.-Q.; He, H.-Y.; Cao, Y. An Aqueous Rechargeable Formate-Based Hydrogen Battery Driven by Heterogeneous Pd Catalysis. Angew. Chem. Int. Ed. 2014, 53, 13583–13587. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, J.; Shao, X.; Su, X.; Huang, Y.; Zhang, T. Palladium on Nitrogen-Doped Mesoporous Carbon: A Bifunctional Catalyst for Formate-Based, Carbon-Neutral Hydrogen Storage. ChemSusChem 2016, 9, 246–251. [Google Scholar] [CrossRef]
- Shao, X.; Xu, J.; Huang, Y.; Su, X.; Duan, H.; Wang, X.; Zhang, T. Pd@C3N4 nanocatalyst for highly efficient hydrogen storage system based on potassium bicarbonate/formate. AIChE J. 2016, 62, 2410–2418. [Google Scholar] [CrossRef]
- Kar, S.; Goeppert, A.; Prakash, G.K.S. Integrated CO2 Capture and Conversion to Formate and Methanol: Connecting Two Threads. Accounts Chem. Res. 2019, 52, 2892–2903. [Google Scholar] [CrossRef]
- Plomp, A.J.; Vuori, H.; Krause, A.O.I.; de Jong, K.P.; Bitter, J.H. Particle size effects for carbon nanofiber supported platinum and ruthenium catalysts for the selective hydrogenation of cinnamaldehyde. Appl. Catal. A Gen. 2008, 351, 9–15. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, S.; Zhang, Q.; Wang, Y. Ruthenium Nanoparticles Supported on Carbon Nanotubes as Efficient Catalysts for Selective Conversion of Synthesis Gas to Diesel Fuel. Angew. Chem. Int. Ed. 2009, 48, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, Y.; Chen, W. PtPd porous nanorods with enhanced electrocatalytic activity and durability for oxygen reduction reaction. Nano Energy 2013, 2, 836–844. [Google Scholar] [CrossRef]
- Chernov, A.N.; Astrakova, T.V.; Koltunov, K.Y.; Sobolev, V.I. Ethanol Dehydrogenation to Acetaldehyde over Co@N-Doped Carbon. Catalysts 2021, 11, 1411. [Google Scholar] [CrossRef]
- Liu, J.; Ji, Y.-G.; Qiao, B.; Zhao, F.; Gao, H.; Chen, P.; An, Z.; Chen, X.; Chen, Y. N,S Co-Doped Carbon Nanofibers Derived from Bacterial Cellulose/Poly(Methylene blue) Hybrids: Efficient Electrocatalyst for Oxygen Reduction Reaction. Catalysts 2018, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, R.; Hävecker, M.; Schlögl, R.; Su, D.S. Dynamic surface rearrangement and thermal stability of nitrogen functional groups on carbon nanotubes. Chem. Commun. 2008, 40, 4891–4893. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, R.; Schuster, M.E.; Xie, Z.; Yi, Y.; Wowsnick, G.; Sun, L.L.; Hermann, K.E.; Friedrich, M.; Kast, P.; Hävecker, M.; et al. Nature of the N–Pd Interaction in Nitrogen-Doped Carbon Nanotube Catalysts. ACS Catal. 2015, 5, 2740–2753. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, J.; Kim, J.Y.; Nam, S.-W.; Han, mJ.H.; Lim, T.-H.; Gautam, S.K.; Chae, H.; Yoon, C.W. Carbon dioxide mediated, reversible chemical hydrogen storage using a Pd nanocatalyst supported on mesoporous graphitic carbon nitride. J. Mater. Chem. A 2014, 2, 9490–9495. [Google Scholar] [CrossRef]
- Elek, J.; Nádasdi, L.; Papp, G.; Laurenczy, G.; Joó, F. Homogeneous hydrogenation of carbon dioxide and bicarbonate in aqueous solution catalyzed by water-soluble ruthenium(II) phosphine complexes. Appl. Catal. A Gen. 2003, 255, 59–67. [Google Scholar] [CrossRef]




| Entry | Capturing and Hydrogenation Solvent | CO2 Loading | Captured CO2 Concentration (M) | Hydrogenation Results of CO2 Species Concentration (M) | Conversion Results | |||
|---|---|---|---|---|---|---|---|---|
| HCO3− | R1R2COO− | HCO3− | R1R2COO− | HCOO− | Formate Yield (%) | |||
| 1 | MEA | 0.15 | 0 | 0.45 | 0 | 0.31 | 0.14 | 30.8 |
| 2 | MEA | 0.31 | 0.14 | 0.79 | 0 | 0.55 | 0.38 | 40.8 |
| 3 | MEA | 0.46 | 0.25 | 1.13 | 0 | 0.63 | 0.75 | 54.1 |
| 4 | MEA | 0.72 | 1.19 | 0.97 | 0 | 0.89 | 1.27 | 58.8 |
| 5 | DEA | 0.16 | 0 | 0.48 | 0 | 0.29 | 0.19 | 35.1 |
| 6 | DEA | 0.31 | 0.17 | 0.76 | 0 | 0.5 | 0.43 | 50.3 |
| 7 | DEA | 0.48 | 0.45 | 0.98 | 0 | 0.71 | 0.72 | 46.3 |
| 8 | DEA | 0.78 | 0.70 | 1.64 | 0 | 1.16 | 1.18 | 60.6 |
| 9 | TEA | 0.15 | 0.45 | 0 | 0.14 | 0 | 0.31 | 68.5 |
| 10 | TEA | 0.30 | 0.9 | 0 | 0.16 | 0 | 0.74 | 82.6 |
| 11 | TEA | 0.46 | 1.38 | 0 | 0.51 | 0 | 0.86 | 62.5 |
| 12 | TEA | 0.60 | 1.8 | 0 | 0.84 | 0 | 0.96 | 53.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, X.; Yao, C.; Xu, M. Integrated CO2 Capture and Hydrogenation to Produce Formate in Aqueous Amine Solutions Using Pd-Based Catalyst. Catalysts 2022, 12, 925. https://doi.org/10.3390/catal12080925
Li L, Chen X, Yao C, Xu M. Integrated CO2 Capture and Hydrogenation to Produce Formate in Aqueous Amine Solutions Using Pd-Based Catalyst. Catalysts. 2022; 12(8):925. https://doi.org/10.3390/catal12080925
Chicago/Turabian StyleLi, Lichun, Xiangcan Chen, Chu Yao, and Meng Xu. 2022. "Integrated CO2 Capture and Hydrogenation to Produce Formate in Aqueous Amine Solutions Using Pd-Based Catalyst" Catalysts 12, no. 8: 925. https://doi.org/10.3390/catal12080925
APA StyleLi, L., Chen, X., Yao, C., & Xu, M. (2022). Integrated CO2 Capture and Hydrogenation to Produce Formate in Aqueous Amine Solutions Using Pd-Based Catalyst. Catalysts, 12(8), 925. https://doi.org/10.3390/catal12080925