Exploring the Effect of Hierarchical Porosity in BEA Zeolite in Friedel-Crafts Acylation of Furan and Benzofuran
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterization
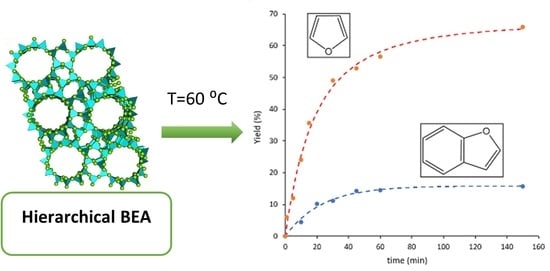
2.2. Results from Catalytic Reactions
2.3. Kinetic Modelling
2.4. Quantitative Structure-Property Relationship Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Hierarchical BEA Zeolite Samples
3.3. Physicochemical Characterization
3.4. Catalytic Experiments
3.5. Kinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Friedel, C.; Crafts, J.M. Sur une nouvelle méthode génerale de de synthése de hydrocarbures, d’acétones, etc. Compt. Rendus 1877, 84, 1392–1395. [Google Scholar]
- Alshorifi, F.T.; Tobbala, D.E.; El-Bahy, S.M.; Nassan, M.A.; Salama, R.S. The role of phosphotungstic acid in enhancing the catalytic performance of UiO-66 (Zr) and its applications as an efficient solid acid catalyst for coumarins and dihydropyrimidinones synthesis. Catal. Commun. 2022, 169, 106479. [Google Scholar] [CrossRef]
- Altass, H.M.; Khder, A.S.; Ahmed, S.A.; Morad, M.; Alsabei, A.A.; Jassas, R.S.; Althagafy, K.; Ahmed, A.I.; Salama, R.S. Highly efficient, recyclable cerium-phosphate solid acid catalysts for the synthesis of tetrahydrocarbazole derivatives by Borsche–Drechsel cyclization. React. Kinet. Mech. Catal. 2021, 134, 143–161. [Google Scholar] [CrossRef]
- Guisnet, M.; Ribeiro, F.R. Les zeolithes: Un Nanomonde au Service de la Catalyse; EDP Science: Les Ullis, France, 2006; ISBN 978286838261. [Google Scholar]
- Clerici, M.G. Zeolites for fine chemicals production. Top. Catal. 2000, 13, 373–386. [Google Scholar] [CrossRef]
- Sartori, G.; Maggi, R. Advances in Friedel-Crafts Acylation Reactions: Catalytic and Green Processes; CRC Press: Boca Raton, FA, USA, 2009; ISBN 9781420067934. [Google Scholar]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal–Organic Frameworks. Adv. Mater. 2017, 29, 1–21. [Google Scholar] [CrossRef]
- Nayak, Y.N.; Nayak, S.; Nadaf, Y.F.; Shetty, N.S.; Gaonkar, S.L. Zeolite Catalyzed Friedel-Crafts Reactions: A Review. Lett. Org. Chem. 2019, 17, 491–506. [Google Scholar] [CrossRef]
- Cavani, F.; Centi, G.; Perathoner, S.; Trifiró, F. Sustainable Industrial Process. In Principles, Tools and Industrial Examples; Wiley: Weinheim, Germany, 2009; ISBN 978-3-527-31552-9. [Google Scholar]
- Carvalho, A.P.; Nunes, N.M.A. Hierarchical Zeolites: Preparation, Properties and Catalytic Applications. In Comprehensive Guide for Mesoporous Materials, Vol. 3: Properties and Development; Aliofkhazraei, M., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 147–211. ISBN 978-1-63463-318-5. [Google Scholar]
- Derouane, E.G.; Schmidt, I.; Lachas, H.; Christensen, C.J.H. Improved performance of nano-size H-BEA zeolite catalysts for the Friedel-Crafts acetylation of anisole by acetic anhydride. Catal. Lett. 2004, 95, 13–17. [Google Scholar] [CrossRef]
- Ji, X.; Qin, Z.; Dong, M.; Wang, G.; Dou, T.; Wang, J. Friedel-Crafts acylation of anisole and toluene with acetic anhydride over nano-sized Beta zeolites. Catal. Lett. 2007, 117, 171–176. [Google Scholar] [CrossRef]
- Paixão, V.; Carvalho, A.P.; Rocha, J.; Fernandes, A.; Martins, A. Modification of MOR by desilication treatments: Structural, textural and acidic characterization. Microporous Mesoporous Mater. 2010, 131, 350–357. [Google Scholar] [CrossRef]
- Fernandez, C.; Stan, I.; Gilson, J.P.; Thomas, K.; Vicente, A.; Bonilla, A.; Pérez-Ramírez, J. Hierarchical ZSM-5 zeolites in shape-selective xylene isomerization: Role of mesoporosity and acid site speciation. Chem. A Eur. J. 2010, 16, 6224–6233. [Google Scholar] [CrossRef]
- Machado, V.; Rocha, J.; Carvalho, A.P.; Martins, A. Modification of MCM-22 zeolite through sequential post-synthesis treatments. Implications on the acidic and catalytic behaviour. Appl. Catal. A Gen. 2012, 445–446, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Verboekend, D.; Mitchell, S.; Milina, M.; Groen, J.C.; Javier, P. Full Compositional Flexibility in the Preparation of Mesoporous MFI Zeolites by Desilication. J. Phys. Chem. 2011, 115, 14193–14203. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Lancelot, C.; Lamonier, C.; Morin, J.C.; Revel, B.; Delevoye, L.; Rives, A. Effect of post treatment on the local structure of hierarchical Beta prepared by desilication and the catalytic performance in Friedel-Crafts alkylation. Microporous Mesoporous Mater. 2015, 206, 42–51. [Google Scholar] [CrossRef]
- Aleixo, R.; Elvas-Leitão, R.; Martins, F.; Carvalho, A.P.; Brigas, A.; Martins, A.; Nunes, N. Kinetic study of Friedel-Crafts acylation reactions over hierarchical MCM-22 zeolites. Mol. Catal. 2017, 434, 175–183. [Google Scholar] [CrossRef]
- Aleixo, R.; Elvas-leitão, R.; Martins, F.; Carvalho, A.P.A.P.; Brigas, A.; Nunes, R.; Fernandes, A.; Rocha, J.; Martins, A.; Nunes, N. Zooming in with QSPR on Friedel-Crafts acylation reactions over modified BEA zeolites. Mol. Catal. 2019, 476, 110495. [Google Scholar] [CrossRef]
- Elvas-Leitão, R.; Martins, F.; Borbinha, L.; Marranita, C.; Martins, A.; Nunes, N. Probing substrate/catalyst effects using QSPR analysis on Friedel-Crafts acylation reactions over hierarchical BEA zeolites. Molecules 2020, 25, 5682. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ansari, L.M.S.; Pombeiro, A.J.L.; Carvalho, A.P.; Martins, A.; Martins, L.M.D.R.S. Fe@hierarchical bea zeolite catalyst for mw-assisted alcohol oxidation reaction: A greener approach. Catalysts 2020, 10, 1029. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Baerlocher, C.H.; Meier, W.M.; Olson, D.H. Atlas of Zeolite Framework Types, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 0444507019. [Google Scholar]
- Guidotti, M.; Coustard, J.-M.; Magnoux, P.; Guisnet, M. Acetylation of aromatics over acid zeolites: Seeking a viable alternative to Friedel-Crafts catalysts. Pure Appl. Chem. 2007, 79, 1833–1838. [Google Scholar] [CrossRef]
- Lu, J.Z.; Acree, W.E.; Abraham, M.H. Updated Abraham model correlations for enthalpies of solvation of organic solutes dissolved in benzene and acetonitrile. Phys. Chem. Liq. 2019, 57, 84–99. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sidney, J.; Sing, K.S.W.; Gregg, S.J.K.S.W.S. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982; ISBN 0-12-300956-1. [Google Scholar]
- Derouane, E.G.; Dillon, C.J.; Bethell, D.; Derouane-Abd Hamid, S.B. Zeolite catalysts as solid solvents in fine chemicals synthesis 1. Catalyst deactivation in the Friedel-Crafts acetylation of anisole. J. Catal. 1999, 187, 209–218. [Google Scholar] [CrossRef]
- Derouane, E.G.; Crehan, G.; Dillon, C.J.; Bethell, D.; He, H.; Derouane-Abd Hamid, S.B. Zeolite catalysts as solid solvents in fine chemicals synthesis: 2. Competitive adsorption of the reactants and products in the Friedel-Crafts acetylations of anisole and toluene. J. Catal. 2000, 194, 410–423. [Google Scholar] [CrossRef]



| Catalyst | CXRD1 (%) | Vmicro2 (cm3 g−1) | Vmeso2 (cm3 g−1) | Aext2 (m2 g−1) | [B]pyr 3 (mmol g−1) T =150 °C T =350 °C | [L]pyr 3(mmol g−1) T =150 °C T =350 °C | ||
|---|---|---|---|---|---|---|---|---|
| BEA | 100 | 0.17 | 0.40 | 246 | 0.225 | 0.078 | 0.273 | 0.212 |
| BEA0.2 | 87 | 0.13 | 0.42 | 211 | 0.238 | 0.105 | 0.231 | 0.176 |
| BEA0.2AT | 90 | 0.15 | 0.49 | 237 | 0.208 | 0.112 | 0.093 | 0.090 |
| BEA0.4 | 76 | 0.12 | 0.43 | 191 | 0.237 | 0.087 | 0.230 | 0.172 |
| BEA0.4AT | 74 | 0.13 | 0.48 | 190 | 0.243 | 0.101 | 0.143 | 0.129 |
| Catalyst | Q4 | Q3 | Q2 | Altet/Aloct |
|---|---|---|---|---|
| BEA | 57 | 33 | 9 | 2.4 |
| BEA0.2 | 53 | 35 | 11 | 2.3 |
| BEA0.2AT | 56 | 32 | 12 | 2.7 |
| BEA0.4 | 50 | 37 | 11 | 2.1 |
| BEA0.4AT | 54 | 35 | 11 | 3.1 |
| Catalyst | Substrate | k (mmol min−1 g−1) | TOF (min−1) | Kr | R2 | sfit | F |
|---|---|---|---|---|---|---|---|
| BEA | Furan | 65 ± 2 | 289 | 8.0 ± 0.6 | 0.992 | 0.275 | 1021 |
| BEA0.2 | Furan | 53 ± 4 | 222 | 7 ± 2 | 0.958 | 0.549 | 160 |
| BEA0.2AT | Furan | 26.4 ± 0.8 | 127 | 4.0 ± 0.5 | 0.990 | 0.142 | 710 |
| BEA0.4 | Furan | 46 ± 1 | 194 | 9.1 ± 0.9 | 0.995 | 0.195 | 663 |
| BEA0.4AT | Furan | 17 ± 2 | 70 | 1.3 ± 0.8 | 0.903 | 0.274 | 56 |
| BEA | Benzofuran | 1.30 ± 0.03 | 5.8 | 25 ± 2 | 0.984 | 0.005 | 494 |
| BEA0.2 | Benzofuran | 1.32 ± 0.06 | 5.5 | 41 ± 5 | 0.959 | 0.009 | 142 |
| BEA0.2AT | Benzofuran | 5.2 ± 0.6 | 25 | 33 ± 8 | 0.905 | 0.083 | 47 |
| BEA0.4 | Benzofuran | 0.95 ± 0.06 | 5.0 | 35 ± 6 | 0.961 | 0.009 | 73 |
| BEA0.4AT | Benzofuran | 2.38 ± 0.08 | 9.8 | 12 ± 2 | 0.962 | 0.016 | 125 |
| Substrate | E | S | A | B | V |
|---|---|---|---|---|---|
| Furan | 0.370 | 0.510 | 0.000 | 0.130 | 0.5363 |
| Benzofuran | 0.890 | 0.830 | 0.000 | 0.150 | 0.9053 |
| Thiophene | 0.687 | 0.570 | 0.000 | 0.150 | 0.6411 |
| Pyrrole | 0.613 | 0.730 | 0.410 | 0.290 | 0.5570 |
| Indole | 1.200 | 1.260 | 0.440 | 0.180 | 0.9464 |
| Anisole | 0.710 | 0.750 | 0.000 | 0.290 | 0.9160 |
| r2 | CXRD | Vmicro | Vmeso | Aext | [B]pyr | [L]pyr | E | S | A | B | V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CXRD | 1.0 | ||||||||||
| Vmicro | 0.89 | 1.0 | |||||||||
| Vmeso | 0.41 | 0.26 | 1.0 | ||||||||
| Aext | 0.95 | 0.91 | 0.21 | 1.0 | |||||||
| [B]pyr | 0.37 | 0.37 | 0.01 | 0.58 | 1.0 | ||||||
| [L]pyr | 0.30 | 0.20 | 0.98 | 0.13 | 0.05 | 1.0 | |||||
| E | 0.03 | 0.04 | 0.03 | 0.03 | 0.00 | 0.03 | 1.0 | ||||
| S | 0.04 | 0.05 | 0.03 | 0.04 | 0.01 | 0.03 | 0.88 | 1.0 | |||
| A | 0.17 | 0.19 | 0.13 | 0.14 | 0.02 | 0.12 | 0.13 | 0.33 | 1.0 | ||
| B | 0.23 | 0.25 | 0.17 | 0.19 | 0.03 | 0.16 | 0.05 | 0.08 | 0.28 | 1.0 | |
| V | 0.01 | 0.01 | 0.11 | 0.01 | 0.00 | 0.00 | 0.85 | 0.68 | 0.01 | 0.05 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, N.; Carvalho, A.P.; Elvas-Leitão, R.; Martins, F.; Fernandes, A.; Rocha, J.; Martins, A. Exploring the Effect of Hierarchical Porosity in BEA Zeolite in Friedel-Crafts Acylation of Furan and Benzofuran. Catalysts 2022, 12, 1064. https://doi.org/10.3390/catal12091064
Nunes N, Carvalho AP, Elvas-Leitão R, Martins F, Fernandes A, Rocha J, Martins A. Exploring the Effect of Hierarchical Porosity in BEA Zeolite in Friedel-Crafts Acylation of Furan and Benzofuran. Catalysts. 2022; 12(9):1064. https://doi.org/10.3390/catal12091064
Chicago/Turabian StyleNunes, Nelson, Ana P. Carvalho, Ruben Elvas-Leitão, Filomena Martins, Auguste Fernandes, João Rocha, and Angela Martins. 2022. "Exploring the Effect of Hierarchical Porosity in BEA Zeolite in Friedel-Crafts Acylation of Furan and Benzofuran" Catalysts 12, no. 9: 1064. https://doi.org/10.3390/catal12091064
APA StyleNunes, N., Carvalho, A. P., Elvas-Leitão, R., Martins, F., Fernandes, A., Rocha, J., & Martins, A. (2022). Exploring the Effect of Hierarchical Porosity in BEA Zeolite in Friedel-Crafts Acylation of Furan and Benzofuran. Catalysts, 12(9), 1064. https://doi.org/10.3390/catal12091064