Highly Active Large Au Clusters and Even More Active Ag Nanoparticles Supported on Ceria-Zirconia: Impact of Particle Size and Potassium Ion Loading on Activity in Catalytic Transfer Hydrogenation
Abstract
:1. Introduction
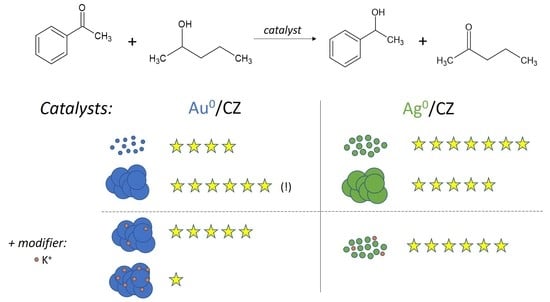
2. Results
3. Materials and Methods
3.1. Support and Catalyst Synthesis
3.2. Support and Catalyst Characterization
3.3. Activity Measurements
4. Conclusions
- Large Au clusters (30 nm) were more active in catalytic transfer hydrogenation than small ones (4 nm).
- Heterogeneous silver catalysts supported on zirconium-doped ceria were highly active in catalytic transfer hydrogenation, whereas those on cerium-doped zirconia showed a much lower activity.
- Au and Ag supported on Ce1−xZrxO2, x < 0.2, were much more active than on Zr1−xCexO2, x < 0.1.
- The effect of potassium ions depended on the loading:
- -
- The zirconium-doped ceria supports showed a positive effect of small doses of potassium ions on the activity in CTH, but negative effects of larger doses.
- -
- The presence of potassium ions on the activity of the most active gold and silver catalysts was negative, even at small loadings.
- Both types of supports contained only one phase: the Ce1−xZrxO2 supports had the fluorite-type structure, typical for undoped ceria and Zr1−xCexO2 supports exhibited the structure of tetragonal zirconia. The activity of the former supports was strongly dependent on Zr loading, whereas that of the latter was not.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 30. [Google Scholar]
- Gliński, M. Catalytic hydrogen transfer over magnesia: Vapour and liquid phase reduction of various aralkyl ketones. Appl. Catal. A Gen. 2008, 349, 133–139. [Google Scholar] [CrossRef]
- Mannu, A.; Grabulosa, A.; Baldino, S. Transfer Hydrogenation from 2-propanol to Acetophenone Catalyzed by [RuCl2(η6-arene)P] (P = monophosphine) and [Rh(PP)2]X (PP = diphosphine, X = Cl−, BF4−) Complexes. Catalysts 2020, 10, 162. [Google Scholar] [CrossRef]
- Vásquez, P.B.; Tabanelli, T.; Monti, E.; Albonetti, S.; Bonincontro, D.; Dimitratos, N.; Cavani, F. Gas-Phase Catalytic Transfer Hydrogenation of Methyl Levulinate with Ethanol over ZrO2. ACS Sustain. Chem. Eng. 2019, 7, 8317–8330. [Google Scholar] [CrossRef]
- Alshammari, A.S. Heterogeneous Gold Catalysis: From Discovery to Applications. Catalysts 2019, 9, 402. [Google Scholar] [CrossRef]
- Meyer, R.; Shaikhutdinov, S.K.; Freund, H.-J. Surface chemistry of catalysis by gold. Gold Bull. 2004, 37, 72–124. [Google Scholar] [CrossRef]
- Calaza, F.; Mahapatra, M.; Neurock, M.; Tysoe, W.T. Disentangling ensemble, electronic and coverage effects on alloy catalysts: Vinyl acetate synthesis on Au/Pd(1 1 1). J. Catal. 2014, 312, 37–45. [Google Scholar] [CrossRef]
- Chen, M.; Kumar, D.; Yi, C.-W.; Goodman, D.W. The promotional effect of gold in catalysis by palladium–gold. Science 2005, 310, 291–293. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon-monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Solsona, B.E.; Garcia, T.; Jones, C.; Taylor, S.H.; Carley, A.F.; Hutchings, G.J. Supported gold catalysts for the total oxidation of alkanes and carbon monoxide. Appl. Catal. A Gen. 2006, 312, 67–76. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M. Supported gold catalysts for CO oxidation and preferential oxidation of CO in H2 stream: Support effect. Catal. Today 2010, 158, 56–62. [Google Scholar] [CrossRef]
- Nkosi, B.; Coville, N.J.; Hutchings, G.J. Reactivation of a supported gold catalyst for acetylene hydrochlorination. J. Chem. Soc. Chem. Commun. 1988, 71–72. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Prati, L.; Rossi, M. Gold on carbon as a new catalyst for selective liquid phase oxidation of diols. J. Catal. 1988, 176, 552–560. [Google Scholar] [CrossRef]
- Flytzani-Stephanopoulos, M. Gold Atoms Stabilized on Various Supports Catalyze the Water-Gas Shift Reaction. Acc. Chem. Res. 2014, 47, 783–792. [Google Scholar] [CrossRef]
- Claus, P.; Brückner, A.; Mohr, C.; Hofmeister, H. Supported Gold Nanoparticles from Quantum Dot to Mesoscopic Size Scale: Effect of Electronic and Structural Properties on Catalytic Hydrogenation of Conjugated Functional Groups. J. Am. Chem. Soc. 2000, 122, 11430–11439. [Google Scholar] [CrossRef]
- Bond, G.C.; Sermon, P.A. Gold catalysts for olefin hydrogenation. Gold Bull. 1973, 6, 102–105. [Google Scholar] [CrossRef]
- Silva, R.J.M.; Fiorio, J.L.; Vidinha, P.; Rossi, L.M. Gold Catalysis for Selective Hydrogenation of Aldehydes and Valorization of Bio-Based Chemical Building Blocks. J. Braz. Chem. Soc. 2019, 30, 2162–2169. [Google Scholar] [CrossRef]
- Schimpf, S.; Lucas, M.; Mohr, C.; Rodemerck, U.; Brückner, A.; Radnik, J.; Hofmeister, C.P. Supported gold nanoparticles: Indepth catalyst characterization and application in hydrogenation and oxidation reactions. Catal. Today 2002, 72, 63–78. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Gorin, D.J.; Toste, F.D. Relativistic Effects in Homogeneous Gold Catalysis. Nature 2007, 446, 395–403. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Zhang, Y.; Zhu, C.; Li, W.; Cheng, Y.; Hu, H. Direct Reductive Amination of Aromatic Aldehydes Catalyzed by Gold(I) Complex under Transfer Hydrogenation Conditions. Chem. Commun. 2011, 47, 6605–6607. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Guan, P.F.; McKenna, K.; Lang, X.Y.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic Origins of the High Catalytic Activity of Nanoporous Gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Su, F.-Z.; He, L.; Ni, J.; Cao, Y.; He, H.-Y.; Fan, K.-N. Efficient and chemoselective reduction of carbonyl compounds with supported gold catalysts under transfer hydrogenation conditions. Chem. Commun. 2008, 3531–3533. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.L.; Lee, H.M. Chemistry of the PCNHCP Ligand: Silver and Ruthenium Complexes, Facial/Meridional Coordination, and Catalytic Transfer Hydrogenation. Organometallics 2005, 24, 1692–1702. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, F.; Jia, Z.; Li, C.-J. A silver-catalyzed transfer hydrogenation of aldehyde in air and water. Org. Chem. Front. 2014, 1, 161–166. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Tsareva, S.; Bruneau, C.; Fischmeister, C. Silver Catalyzed Hydrogenation of Ketones under Mild Conditions. Adv. Synth. Catal. 2019, 361, 786–790. [Google Scholar] [CrossRef]
- Li, A.Y.; Kaushik, M.; Li, C.J.; Moores, A. Microwave-assisted synthesis of magnetic carboxymethyl cellulose-embedded Ag-Fe3O4 nanocatalysts for selective carbonyl hydrogenation. ACS Sustain. Chem. Eng. 2016, 4, 965–973. [Google Scholar] [CrossRef]
- Mertens, P.G.N.; Vandezande, P.; Ye, X.; Poelman, H.; Vankelekom, I.F.J.; De Vos, D.E. Recyclable Au0, Ag0 and Au0–Ag0 nanocolloids for the chemoselective of α,β-unsaturated aldehydes and ketones to allylic alcohols. Appl. Catal. A Gen. 2009, 355, 176–183. [Google Scholar] [CrossRef]
- Claus, P.; Hofmeister, H. Electron Microscopy and Catalytic Study of Silver Catalysts: Structure Sensitivity of the Hydrogenation of Crotonaldehyde. J. Phys. Chem. B 1999, 103, 2766–2775. [Google Scholar] [CrossRef]
- Deng, X.; Li, M.; Zhang, J.; Hu, X.; Zheng, J.; Zhang, N.; Chen, B.H. Constructing nano-structure on silver/ceria-zirconia towards highly active and stable catalyst for soot oxidation. Chem. Eng. J. 2017, 313, 544–555. [Google Scholar] [CrossRef]
- Liens, A.; Reveron, H.; Douillard, T.; Blanchard, N.; Lughi, V.; Sergo, V.; Laquai, R.; Müller, B.R.; Bruno, G.; Schomer, S.; et al. Phase transformation induces plasticity with negligible damage in ceria-stabilized zirconia-based ceramics. Acta Mater. 2019, 183, 261–273. [Google Scholar] [CrossRef]
- Tsukuma, K.; Shimada, M. Strength, fracture toughness and Vickers hardness of CeO2-stabilized tetragonal ZrO2 polycrystals (Ce-TZP). J. Mater. Sci. 1985, 20, 1178–1184. [Google Scholar] [CrossRef]
- El Attaoui, H.; Saâdaoui, M.; Chevalier, J.; Fantozzi, G. Static and cyclic crack propagation in Ce-TZP ceramics with different amounts of transformation toughening. J. Eur. Ceram. Soc. 2007, 27, 483–486. [Google Scholar] [CrossRef]
- Querino, P.S.; Bispo, J.R.C.; Rangel, M.C. The effect of cerium on the properties of Pt/ZrO2 catalysts in the WGSR. Catal. Today 2005, 107–108, 920–925. [Google Scholar] [CrossRef]
- Morozova, L.V.; Drozdova, I.A. Synthesis of Dispersed Mesoporous Powders of Solid Solution Zr0.88Ce0.12O2 for Catalyst Carrier of the Conversion of Methane to Synthesis-Gas. Inorg. Mater. Appl. Res. 2020, 11, 1244–1252. [Google Scholar] [CrossRef]
- Lu, H.-F.; Zhou, Y.; Han, W.-F.; Huang, H.-F.; Chen, Y.-F. High thermal stability of ceria-based mixed oxide catalysts supported on ZrO2 for toluene combustion. Catal. Sci. Technol. 2013, 3, 1480. [Google Scholar] [CrossRef]
- Sohn, J.R.; Lee, S.H.; Lim, J.S. New solid superacid catalyst prepared by doping ZrO2 with Ce and modifying with sulfate and its catalytic activity for acid catalysis. Catal. Today 2006, 116, 143–150. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. The poisoning effect of alkali metals doping over nano V2O5–WO3/TiO2 catalysts on selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Bion, N.; Hamada, H. Alkali metal-doped cobalt oxide catalysts for NO decomposition. Appl. Catal. B Environ. 2003, 46, 473–482. [Google Scholar] [CrossRef]
- Pacultová, K.; Klegova, A.; Karásková, K.; Fridrichová, D.; Bílková, T.; Koštejn, M.; Obalová, L. Oxygen effect in NO direct decomposition over K/Co-Mg-Mn-Al mixed oxide catalyst–Temperature programmed desorption study. J. Mol. Catal. 2021, 510, 111695. [Google Scholar] [CrossRef]
- Sun, H.; Qiu, P.; Yang, Y.; Liu, J.; Zhang, L.; Liu, Z. Molecular insights into the vinyl acetate synthesis on Pd/Au (111) Surface: Effects of potassium ion. Appl. Surf. Sci. 2022, 571, 151297. [Google Scholar] [CrossRef]
- Parida, K.; Mishra, H.K. Catalytic ketonisation of acetic acid over modified zirconia. J. Mol. Catal. A Chem. 1999, 139, 73–80. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; He, H.; Zhang, J.; Chen, M. Influence of alkali metals on Pd/TiO2 catalysts for catalytic oxidation of formaldehyde at room temperature. Catal. Sci. Technol. 2016, 6, 2289–2295. [Google Scholar] [CrossRef]
- Iwanek, E.; Liotta, L.F.; Williams, S.; Hu, L.; Calilung, L.F.; Pantaleo, G.; Kaszkur, Z.; Kirk, D.W.; Gliński, M. Application of Potassium Ion Deposition in Determining the Impact of Support Reducibility on Catalytic Activity of Au/Ceria-Zirconia Catalysts in CO Oxidation, NO Oxidation, and C3H8 Combustion. Catalysts 2020, 10, 688. [Google Scholar] [CrossRef]
- Iwanek, E.M.; Liotta, L.F.; Williams, S.; Hu, L.; Ju, H.; Pantaleo, G.; Thapar, A.; Kaszkur, Z.; Kirk, D.W.; Gliński, M. Activity of Ag/CeZrO2, Ag+K/CeZrO2, and Ag-Au+K/CeZrO2 Systems for Lean Burn Exhaust Clean-Up. Catalysts 2021, 11, 1041. [Google Scholar] [CrossRef]
- Iwanek (nee Wilczkowska), E.M.; Liotta, L.F.; Williams, S.; Hu, L.; Ju, H.; Pantaleo, G.; Kaszkur, Z.; Kirk, D.W.; Patkowski, W.; Gliński, M. Reducibility Studies of Ceria, Ce0.85Zr0.15O2 (CZ) and Au/CZ Catalysts after Alkali Ion Doping: Impact on Activity in Oxidation of NO and CO. Catalysts 2022, 12, 524. [Google Scholar] [CrossRef]
- Kowalik, P.; Próchniak, W.; Borowiecki, T. The effect of alkali metals doping on properties of Cu/ZnO/Al2O3 catalyst for water gas shift. Catal. Today 2011, 176, 144–148. [Google Scholar] [CrossRef]
- Shah, P.M.; Burnett, J.W.H.; Morgan, D.J.; Davies, T.E.; Taylor, S.H. Ceria–Zirconia Mixed Metal Oxides Prepared via Mechanochemical Grinding of Carbonates for the Total Oxidation of Propane and Naphthalene. Catalysts 2019, 9, 475. [Google Scholar] [CrossRef] [Green Version]









| Sample | Surface Area [m2/g] | Particle Size [nm] 1 | Cell Parameter(s) [nm] | Structure; ε 2 |
|---|---|---|---|---|
| CeO2 | 52.8 | 6.0(8) | 0.5408(2) | cubic; 0.002 |
| Ce0.90Zr0.10O2 | 95.2 | 6.5(8) | 0.5374(2) | cubic; 0.003 |
| Ce0.85Zr0.15O2 | 96.0 | 5.1(8) | 0.5362(2) | cubic; 0.002 |
| Ce0.80Zr0.20O2 | 87.2 | 5.7(8) | 0.5350(2) | cubic; 0.002 |
| ZrO2 | 48.0 | 10.7(8), 7.1(8) | n.d. | monoclinic, cubic |
| Zr0.93Ce0.07O2 | 63.1 | 7.0(8) | a = 0.364(1), c = 0.523(1) | tetragonal |
| Zr0.90Ce0.10O2 | 62.9 | 7.0(8) | a = 0.363(1), c = 0.523(1) | tetragonal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanek, E.M.; Gliński, M.; Siwiec, A.; Siennicka, S.; Zybert, M.; Kaszkur, Z. Highly Active Large Au Clusters and Even More Active Ag Nanoparticles Supported on Ceria-Zirconia: Impact of Particle Size and Potassium Ion Loading on Activity in Catalytic Transfer Hydrogenation. Catalysts 2022, 12, 974. https://doi.org/10.3390/catal12090974
Iwanek EM, Gliński M, Siwiec A, Siennicka S, Zybert M, Kaszkur Z. Highly Active Large Au Clusters and Even More Active Ag Nanoparticles Supported on Ceria-Zirconia: Impact of Particle Size and Potassium Ion Loading on Activity in Catalytic Transfer Hydrogenation. Catalysts. 2022; 12(9):974. https://doi.org/10.3390/catal12090974
Chicago/Turabian StyleIwanek (nee Wilczkowska), Ewa M., Marek Gliński, Aleksandra Siwiec, Sylwia Siennicka, Magdalena Zybert, and Zbigniew Kaszkur. 2022. "Highly Active Large Au Clusters and Even More Active Ag Nanoparticles Supported on Ceria-Zirconia: Impact of Particle Size and Potassium Ion Loading on Activity in Catalytic Transfer Hydrogenation" Catalysts 12, no. 9: 974. https://doi.org/10.3390/catal12090974
APA StyleIwanek, E. M., Gliński, M., Siwiec, A., Siennicka, S., Zybert, M., & Kaszkur, Z. (2022). Highly Active Large Au Clusters and Even More Active Ag Nanoparticles Supported on Ceria-Zirconia: Impact of Particle Size and Potassium Ion Loading on Activity in Catalytic Transfer Hydrogenation. Catalysts, 12(9), 974. https://doi.org/10.3390/catal12090974