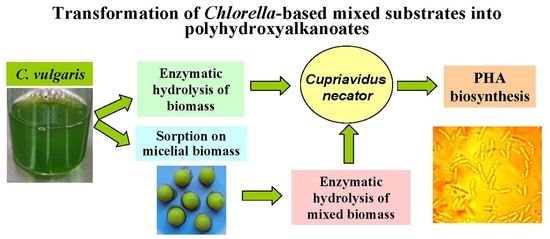
Transformation of Enzymatic Hydrolysates of Chlorella–Fungus Mixed Biomass into Poly(hydroxyalkanoates)
Abstract
:1. Introduction
2. Results
2.1. Comparison of the Effectiveness of Various Disintegration Methods of Microalgae C. vulgaris Biomass and Subsequent Conversion of the Obtained Hydrolysates into PHA
2.2. Biosorption of Microalgae Cells by the Spent Fungal Mycelium, Its Characterization, and Disintegration
2.3. Biosynthesis of PHA from the Mixed Biomass and Characterization of the Polymer
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Their Cultivation
4.2. Disintegration of Biomass and Its Use for PHA Biosynthesis
4.3. Analytical Methods
4.4. Calculation of Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoang, A.T.; Sirohi, R.; Pandey, A.; Nižetić, S.; Lam, S.S.; Chen, W.H.; Luque, R.; Thomas, S.; Arıcı, M.; Pham, V.V. Biofuel production from microalgae: Challenges and chances. Phytochem. Rev. 2022. [Google Scholar] [CrossRef]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Senko, O.V.; Makhlis, T.A.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeyev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef]
- Senko, O.; Maslova, O.; Gladchenko, M.; Gaydamaka, S.; Efremenko, E. Biogas production from biomass of microalgae Chlorella vulgaris in the presence of benzothiophene sulfone. IOP Conf. Ser. Mater. Sci. Eng. 2019, 525, 012089. [Google Scholar] [CrossRef] [Green Version]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Eseva, E.; Anisimov, A.; Efremenko, E. Sulfur containing mixed wastes in anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Maslova, O.; Stepanov, N.; Aslanli, A.; Lyagin, I. Biocatalysts in synthesis of microbial polysaccharides: Properties and development trends. Catalysts 2022, 12, 1377. [Google Scholar] [CrossRef]
- Tan, F.; Nadir, N.; Sudesh, K. Microalgal biomass as feedstock for bacterial production of PHA: Advances and future prospects. Front. Bioeng. Biotechnol. 2022, 10, 879476. [Google Scholar] [CrossRef]
- Khomlaem, C.; Aloui, H.; Kim, B.S. Biosynthesis of polyhydroxyalkanoates from defatted Chlorella biomass as an inexpensive substrate. Appl. Sci. 2021, 11, 1094. [Google Scholar] [CrossRef]
- Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Delerue-Matos, C. Microplastic pollution focused on sources, distribution, contaminant interactions, analytical methods, and wastewater removal strategies: A review. Int. J. Environ. Res. Public Health 2022, 19, 5610. [Google Scholar] [CrossRef]
- Coppola, G.; Gaudio, M.T.; Lopresto, C.G.; Calabro, V.; Curcio, S.; Chakraborty, S. Bioplastic from renewable biomass: A facile solution for a greener environment. Earth Syst. Environ. 2021, 5, 231–251. [Google Scholar] [CrossRef]
- Lyagin, I.; Maslova, O.; Stepanov, N.; Presnov, D.; Efremenko, E. Assessment of composite with fibers as a support for antibacterial nanomaterials: A case study of bacterial cellulose, polylactide and usual textile. Fibers 2022, 10, 70. [Google Scholar] [CrossRef]
- Koller, M. Review: Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [Green Version]
- Brojanigo, S.; Gronchi, N.; Cazzorla, T.; Wong, T.S.; Basaglia, M.; Favaro, L.; Casella, S. Engineering Cupriavidus necator DSM 545 for the one-step conversion of starchy waste into polyhydroxyalkanoates. Bioresour. Technol. 2022, 347, 126383. [Google Scholar] [CrossRef]
- Tan, H.T.; Chek, M.F.; Lakshmanan, M.; Foong, C.P.; Hakoshima, T.; Sudesh, K. Evaluation of BP-M-CPF4 polyhydroxyalkanoate (PHA) synthase on the production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from plant oil using Cupriavidus necator transformants. Int. J. Biol. Macromol. 2020, 159, 250–257. [Google Scholar] [CrossRef]
- Zainab-L, I.; Uyama, H.; Li, C.; Shen, Y.; Sudesh, K. Production of polyhydroxyalkanoates from underutilized plant oils by Cupriavidus necator. CLEAN–Soil Air Water 2018, 46, 1700542. [Google Scholar] [CrossRef]
- Purama, R.K.; Al-Sabahi, J.N.; Sudesh, K. Evaluation of date seed oil and date molasses as novel carbon sources for the production of poly (3Hydroxybutyrate-co-3Hydroxyhexanoate) by Cupriavidus necator H16 Re 2058/pCB113. Ind. Crops Prod. 2018, 119, 83–92. [Google Scholar] [CrossRef]
- Thinagaran, L.; Sudesh, K. Evaluation of sludge palm oil as feedstock and development of efficient method for its utilization to produce polyhydroxyalkanoate. Waste Biomass Valor. 2019, 10, 709–720. [Google Scholar] [CrossRef]
- Domingos, J.M.; Puccio, S.; Martinez, G.A.; Amaral, N.; Reis, M.A.; Bandini, S.; Fava, F.; Bertin, L. Cheese whey integrated valorisation: Production, concentration and exploitation of carboxylic acids for the production of polyhydroxyalkanoates by a fed-batch culture. Chem. Eng. J. 2018, 336, 47–53. [Google Scholar] [CrossRef]
- Johnston, B.; Radecka, I.; Hill, D.; Chiellini, E.; Ilieva, V.I.; Sikorska, W.; Musiol, M.; Zieba, M.; Marek, A.A.; Keddie, D.; et al. The microbial production of polyhydroxyalkanoates from waste polystyrene fragments attained using oxidative degradation. Polymers 2018, 10, 957. [Google Scholar] [CrossRef] [Green Version]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Grape winery waste as a promising feedstock for the production of polyhydroxyalkanoates and other value-added products. Food Bioprod. Process. 2020, 124, 1–10. [Google Scholar] [CrossRef]
- Vu, D.H.; Mahboubi, A.; Root, A.; Heinmaa, I.; Taherzadeh, M.J.; Åkesson, D. Thorough investigation of the effects of cultivation factors on polyhydroalkanoates (PHAs) production by Cupriavidus necator from food waste-derived volatile fatty acids. Fermentation 2022, 8, 605. [Google Scholar] [CrossRef]
- Altun, M. Polyhydroxyalkanoate production using waste vegetable oil and filtered digestate liquor of chicken manure. Prep. Biochem. Biotechnol. 2019, 49, 493–500. [Google Scholar] [CrossRef]
- Muhammad, M.; Aloui, H.; Khomlaem, C.; Hou, C.T.; Kim, B.S. Production of polyhydroxyalkanoates and carotenoids through cultivation of different bacterial strains using brown algae hydrolysate as a carbon source. Biocatal. Agric. Biotechnol. 2020, 30, 101852. [Google Scholar] [CrossRef]
- Azizi, N.; Najafpour, G.; Younesi, H. Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (PHB) production using Cupriavidus necator. Int. J. Biol. Macromol. 2017, 101, 1029–1040. [Google Scholar] [CrossRef]
- Tu, W.; Chu, H.; Huang, C.; Chen, C.; Ou, C.; Guo, G. Polyhydroxyalkanoate production by Cupriavidus necator with inedible rice. BioResources 2022, 17, 2202–2213. [Google Scholar] [CrossRef]
- Sarmiento-Vásquez, Z.; Vandenberghe, L.P.D.S.; Karp, S.G.; Soccol, C.R. Production of polyhydroxyalkanoates through soybean hull and waste glycerol valorization: Subsequent alkaline pretreatment and enzymatic hydrolysis. Fermentation 2022, 8, 433. [Google Scholar] [CrossRef]
- Gahlawat, G.; Kumar Soni, S. Study on sustainable recovery and extraction of Polyhydroxyalkanoates (PHAs) produced by Cupriavidus necator using waste glycerol for medical applications. Chem. Biochem. Eng. Q. 2019, 33, 99–110. [Google Scholar] [CrossRef]
- Khomlaem, C.; Aloui, H.; Deshmukh, A.R.; Yun, J.-H.; Kim, H.-S.; Napathorn, S.C.; Kim, B.S. Defatted Chlorella biomass as a renewable carbon source for polyhydroxyalkanoates and carotenoids co-production. Algal Res. 2020, 51, 102068. [Google Scholar] [CrossRef]
- De Bari, I.; Giuliano, A.; Petrone, M.T.; Stoppiello, G.; Fatta, V.; Giardi, C.; Razza, F.; Novelli, A. From Cardoon Lignocellulosic Biomass to Bio-1,4 Butanediol: An Integrated Biorefinery Model. Processes 2020, 8, 1585. [Google Scholar] [CrossRef]
- Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. “Nature-like” cryoimmobilization of phototrophic microorganisms: New opportunities for their long-term storage and sustainable use. Sustainability 2022, 14, 661. [Google Scholar] [CrossRef]
- Ruoyu, C.; Shuangxi, L.; Liandong, Z.; Zhihong, Y.; Dan, H.; Chenchen, L.; Fan, M. A review on co-cultivation of microalgae with filamentous fungi: Efficient harvesting, wastewater treatment and biofuel production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Jiang, J.; Jin, W.; Tu, R.; Han, S.; Ji, Y.; Zhou, X. Harvesting of microalgae Chlorella pyrenoidosa by bio-flocculation with bacteria and filamentous fungi. Waste Biomass Valorization 2021, 12, 145–154. [Google Scholar] [CrossRef]
- Zamalloa, C.; Gultom, S.O.; Rajendran, A.; Hu, B. Ionic effects on microalgae harvest via microalgae-fungi co-pelletization. Biocatal. Agric. Biotechnol. 2017, 9, 145–155. [Google Scholar] [CrossRef]
- Alrubaie, G.; Al-Shammari, R.H. Microalgae Chlorella vulgaris harvesting via co-pelletization with filamentous fungus. Baghd. Sci. J. 2018, 15, 31–36. [Google Scholar] [CrossRef]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Liu, J.; Chen, P.; Ruan, R.; Zhou, W. A comparative study between fungal pellet-and spore-assisted microalgae harvesting methods for algae bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Wang, Q. A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 2019, 275, 35–43. [Google Scholar] [CrossRef]
- Wang, S.K.; Yang, K.X.; Zhu, Y.R.; Zhu, X.Y.; Nie, D.F.; Jiao, N.; Angelidaki, I. One-step co-cultivation and flocculation of microalgae with filamentous fungi to valorize starch wastewater into high-value biomass. Bioresour. Technol. 2022, 361, 127625. [Google Scholar] [CrossRef]
- Zorn, S.M.; Reis, C.E.; Silva, M.B.; Hu, B.; De Castro, H.F. Consortium growth of filamentous fungi and microalgae: Evaluation of different cultivation strategies to optimize cell harvesting and lipid accumulation. Energies 2020, 13, 3648. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, L.; Li, P.; Yan, Y.; Chen, T.; Zheng, T.; Wang, H. Flocculation mechanism of Aspergillus niger on harvesting of Chlorella vulgaris biomass. Algal Res. 2017, 25, 402–412. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mathur, M.; Kumar, P.; Prajapati, S.K.; Malik, A. A rapid method for fungal assisted algal flocculation: Critical parameters & mechanism insights. Algal Res. 2017, 21, 42–51. [Google Scholar] [CrossRef]
- Lal, A.; Banerjee, S.; Das, D. Aspergillus sp. assisted bioflocculation of Chlorella MJ 11/11 for the production of biofuel from the algal-fungal co-pellet. Sep. Purif. Technol. 2021, 272, 118320. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Q.; Cui, L.; Cheng, J.; Zhou, H.; Zhang, Y.; Peng, A.; Shen, L. Synergism and mutualistic interactions between microalgae and fungi in fungi-microalgae symbiotic system. Bioresour. Technol. 2022, 361, 127728. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Ugarova, N.N.; Lomakina, G.Y.; Senko, O.V.; Stepanov, N.A.; Maslova, O.V.; Aslanly, A.G.; Lyagin, I.V. Bioluminescent ATP-Metry: Practical Aspects; Scientific Library: Moscow, Russia, 2022; p. 376. ISBN 978-5-907497-77-1. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Zubaerova, D.; Podorozhko, E.; Lozinsky, V. New biocatalyst with multiple enzymatic activities for treatment of complex food wastewater. Food Technol. Biotechnol. 2008, 46, 208–212. [Google Scholar]
- Efremenko, E.N.; Stepanov, N.A.; Gudkov, D.A.; Senko, O.V.; Lozinsky, V.I.; Varfolomeev, S.D. Immobilized fungal biocatalysts for the production of cellulase complex hydrolyzing renewable plant feedstock. Catal. Ind. 2013, 5, 190–198. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Dotsenko, G.S.; Senko, O.V.; Stepanov, N.A.; Lyagin, I.V.; Efremenko, E.N.; Gusakov, A.V.; Zorov, I.N.; Rubtsova, E.A. Complex effect of lignocellulosic biomass pretreatment with 1-butyl-3-methylimidazolium chloride ionic liquid on various aspects of ethanol and fumaric acid production by immobilized cells within SSF. Bioresour. Technol. 2018, 250, 429–438. [Google Scholar] [CrossRef]
- Canelli, G.; Murciano Martίnez, P.; Austin, S.; Ambühl, M.E.; Dionisi, F.; Bolten, C.J.; Caprine, R.; Neutsh, L.; Mathys, A. Biochemical and morphological characterization of heterotrophic Crypthecodinium cohnii and Chlorella vulgaris cell walls. J. Agric. Food Chem. 2021, 69, 2226–2235. [Google Scholar] [CrossRef]
- Efremenko, E.N. Immobilized Cells: Biocatalysts and Processes; RIOR: Moscow, Russia, 2018; p. 524. ISBN 978-5-369-02004-3. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, Y.; Gong, X.; Wang, Q.; Ma, Y. Ionic liquids-based hydrolysis of Chlorella biomass for fermentable sugars. Bioresour. Technol. 2012, 118, 512–517. [Google Scholar] [CrossRef]
- Miranda, J.R.; Passarinho, P.C.; Gouveia, L. Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour. Technol. 2012, 104, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hu, B. A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour. Technol. 2012, 114, 529–535. [Google Scholar] [CrossRef]
- Kshirsagar, P.R.; Kulkarni, S.O.; Nilegaonkar, S.S.; Niveditha, M.; Kanekar, P.P. Kinetics and model building for recovery of polyhydroxyalkanoate (PHA) from Halomonas campisalis. Separ. Purif. Technol. 2013, 103, 151–160. [Google Scholar] [CrossRef]
- Dubber, D.; Gray, N.F. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2010, 45, 1595–1600. [Google Scholar] [CrossRef]





| Substrate [Reference] | Dry Cell Weight (DCW) (g/L) | PHA Concentration (g/L) | Intracellular PHA Content (%) | * QPHA (mg/L/h) |
|---|---|---|---|---|
| Broken rice waste [13] | 14.0 | 47.3 | 6.5 | 67.8 |
| Palm oil [14] | 4.7 | 4.0 | 83.7 | 83.3 |
| Desert date oil [15] | 9.0 | 40 | 3.6 | 75.0 |
| Date molasses and date seed oil combination [16] | 6.9 | 51.0 | 3.5 | 72.9 |
| Sludge palm oil [17] | 9.7 | 74.0 | 7.2 | 150.0 |
| Carboxylic acids obtained by the anaerobic fermentation of cheese whey [18] | 15.0 | 71.0 | 10.0 | 192.3 |
| Polystyrene waste pretreated with thermal oxidation processes [19] | 3.6 | 48.0 | 1.7 | 35.8 |
| Grape winery waste [20] | 8.3 | 63.0 | 5.2 | 176.3 |
| Food waste-derived volatile fatty acids [21] | 2.2 | 75.3 | 1.6 | 44.4 |
| Digestate of chicken manure combined with waste sunflower oil [22] | 6.1 | 75.1 | 4.6 | 47.9 |
| Laminaria japonica biomass acid hydrolysate [23] | 5.0 | 32 | 1.6 | 26.3 |
| Sargassum sp. biomass enzymatic hydrolysate [24] | 5.4 | 74.4 | 3.9 | 109.2 |
| The hydrolysis liquid from inedible rice [25] | 7.0 | 68.6 | 4.8 | 66.9 |
| Soybean hull enzymatic hydrolysate with waste glycerol [26] | 6.7 | 39.0 | 2.6 | 27.1 |
| Waste glycerol [27] | 10.5 | 69.0 | 7.3 | 183.0 |
| Acid hydrolysate of defatted Chlorella biomass [8,28] | 10.0 | 75.4 | 7.5 | 62.6 |
| Microalgae/Fungi (M/F) [Reference] | Process Conditions | Harvesting Efficiency, % |
|---|---|---|
| Spore-assisted harvesting | ||
| C. pyrenoidosa/Mucor circinelloides [32] | Ratio M/F = 333:1, 24 h, 150 rpm, 1.5 g/L glucose | ~100 |
| C. vulgaris/Aspergillus niger [33] | Ratio M/F = 300:1, 72 h, 150 rpm, 2 g/L glucose | >90 |
| C. vulgaris/A. niger [34] | 1×104 spore/mL, 72 h, 150 rpm, 15 g/L sucrose | ~100 |
| Chlorella sp./Penicillium sp. [35] | 1×104 spore/mL, 28 h, 160 rpm, 5 g/L glucose | 99 |
| C. vulgaris/Aspergillus sp. [36] | Ratio M/F = 100:1, 4 h, 80 rpm, molasses wastewater | 97 |
| C. pyrenoidosa/A. oryzae [37] | Ratio M/F = 4000:1, 72 h, 150 rpm, 5.5 g/L total sugars | ~100 |
| C. vulgaris/M. circinelloides [38] | Ratio M/F = 300:1, 180 h, 140 rpm, 2 g/L glucose | 95 |
| Spore-assisted harvesting with support matrix | ||
| C. vulgaris/M. circinelloides [38] | Ratio M/F = 300:1, 180 h, 140 rpm, 2 g/L glucose, cotton patches (2.5 cm × 2.5 cm) as support | ~100 |
| Pellet-assisted harvesting | ||
| Chlorella sp./Penicillium sp. [34] | Ratio M/F= 2:1, 2.5 h, 160 rpm | 98 |
| C. vulgaris/Aspergillus sp. [36] | 2 g dry weight fungal biomass/L, 4 h, molasses wastewater | 97 |
| C. vulgaris/A. niger [39] | Ratio M/F = (4.8 × 1010 cells/mL): (4 fungal pellets (each ~1 cm)/10 mL), 24 h, 120 rpm | 93 |
| C. pyrenoidosa/A. fumigatus [40] | Ratio M/F = 5:1, 3 h, 100 rpm | 99 |
| Chlorella sp./Aspergillus sp. [41] | Ratio M/F = 1:3, 5 h, 100 rpm, 6 g/L dextrose | >90 |
| Conditions of Biomass Disintegration | * CRS, g/L | CGLU, g/L | QRS, g/L/h | QGLU, g/L/h |
|---|---|---|---|---|
| CDCW = 20 g/L | ||||
| Acid hydrolysis combined with thermolysis (1.2 M HCl, 0.5 h, 121 °C, 1 atm) | 6.9 ± 0.3 | 2.3 ± 0.07 | 13.8 ± 0.6 | 4.6 ± 0.2 |
| Acid hydrolysis combined with thermolysis (1.0 M H2SO4, 0.75 h, 121 °C, 1 atm) | 7.7 ± 0.3 | 3.4 ± 0.08 | 10.2 ± 0.5 | 4.5 ± 0.2 |
| Mechanical destruction (4 min) and further acid hydrolysis combined with thermolysis (1.0 M H2SO4, 0.75 h, 121 °C, 1 atm) | 8.5 ± 0.3 | 4.6 ± 0.1 | 11.3 ± 0.5 | 6.1 ± 0.3 |
| Enzymatic hydrolysis (20 h, 37 °C, pH 5.5) ** | 3.0 ± 0.1 | 1.4 ± 0.04 | 0.2 ± 0.01 | 0.07 ± 0.01 |
| Thermolysis (0.5 h, 108 °C, 0.5 atm) and further enzymatic hydrolysis (20 h, 37 °C, pH 5.5) ** | 8.4 ± 0.3 | 5.9 ± 0.2 | 0.4 ± 0.02 | 0.3 ± 0.01 |
| Treatment by ionic liquid [Bmim]Cl (1 h, 120 °C) and further enzymatic hydrolysis (20 h, 37 °C, pH 5.5) ** | 5.7 ± 0.2 | 3.8 ± 0.1 | 0.3 ± 0.01 | 0.2 ± 0.01 |
| Mechanical destruction (4 min) and further enzymatic hydrolysis (20 h, 37 °C, pH 5.5) ** | 9.8 ± 0.3 | 7.8 ± 0.2 | 0.5 ± 0.02 | 0.4 ± 0.01 |
| CDCW = 30 g/L | ||||
| Mechanical destruction (4 min) and Enzymatic hydrolysis (20 h, 37 °C, pH 5.5) ** | 14.7 ± 0.8 | 11.6 ± 0.6 | 0.7 ± 0.03 | 0.6 ± 0.02 |
| CDCW = 50 g/L | ||||
| Mechanical destruction (4 min) and Enzymatic hydrolysis (20 h, 37 °C, pH 5.5) ** | 24.2 ± 1.2 | 19.1 ± 0.9 | 1.2 ± 0.06 | 1.0 ± 0.04 |
| CDCW = 70 g/L | ||||
| Mechanical destruction (4 min) and Enzymatic hydrolysis (20 h, 37 °C, pH 5.5) ** | 33.1 ± 1.6 | 25.0 ± 1.2 | 1.7 ± 0.08 | 1.3 ± 0.06 |
| CDCW = 100 g/L | ||||
| Mechanical destruction (4 min) and Enzymatic hydrolysis (20 h, 37 °C, pH 5.5) ** | 39.4 ± 1.8 | 28.3 ± 1.4 | 2.0 ± 0.09 | 1.4 ± 0.07 |
| Initial Concentration of C. vulgaris Biomass, Used for Mechanical–Enzymatic Disintegration, g DCW/L | 30 | 50 | 70 |
|---|---|---|---|
| Duration of process, h | 10 | 15 | 20 |
| Intracellular PHA content, % | 51 ± 1.3 | 60 ± 1.8 | 59 ± 1.7 |
| Average rate of cell biomass accumulation, QCB, mg DCW/L/h | 396 ± 10 | 440 ± 13 | 320 ± 9 |
| Average rate of PHA accumulation, QPHA, mg/L/h | 373 ±10 | 405 ± 12 | 294 ± 8 |
| * Type of Biomass | Lipids, % | Proteins, % | Carbohydrates, % |
|---|---|---|---|
| Biomass of individual cultures | |||
| Biomass of C. vulgaris cells | 17.1 ± 0.9 | 9.9 ± 0.5 | 55.5 ± 2.5 |
| Immobilized mycelium of R. oryzae F1032 after fumaric acid production | 8.0 ± 0.8 | 22.1 ± 1.1 | 46.8 ± 2.3 |
| Immobilized mycelium of R. oryzae F1814 after food wastewater treatment | 46.1 ± 2.3 | 16.1 ± 0.8 | 24.1 ± 1.2 |
| Immobilized mycelium of A. terreus F728 after enzyme production | 5.1 ± 0.2 | 37.2 ± 1.8 | 39.7 ± 1.9 |
| Mixed biomass | |||
| Immobilized mycelium of R. oryzae F1032 with sorbed biomass of C. vulgaris | 13.8 ± 0.6 | 13.6 ± 0.6 | 52.3 ± 2.6 |
| Immobilized mycelium of R. oryzae F814 after food wastewater treatment with sorbed biomass of C. vulgaris | 25.7 ± 1.2 | 10.7 ± 0.5 | 44.7 ± 2.2 |
| Immobilized mycelium of A. terreus F728 fungi after enzyme production with sorbed biomass of C. vulgaris | 12.5 ± 0.6 | 18.4 ± 0.9 | 49.7 ± 1.9 |
| Medium with Biomass Content | * CRS, g/L | CGLU, g/L | QRS, g/L/h | QGLU, g/L/h | COD, g/L |
|---|---|---|---|---|---|
| Immobilized R. oryzae F1032 after the fumaric acid production with sorbed biomass of C. vulgaris | 21.91 ± 1.09 | 13.02 ± 0.65 | 1.10 ± 0.05 | 0.65 ± 0.03 | 55.3 ± 2.7 |
| Immobilized R. oryzae F814 after use in food wastewater treatment with sorbed C. vulgaris cells | 17.59 ± 0.87 | 8.79 ± 0.43 | 0.88 ± 0.04 | 0.44 ± 0.02 | 70.2 ± 3.51 |
| Immobilized A. terreus F728 fungi after production of enzymes with sorbed C. vulgaris cells | 19.56 ± 0.97 | 9.27 ± 0.46 | 0.98 ± 0.04 | 0.46 ± 0.02 | 53.4 ± 2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. Transformation of Enzymatic Hydrolysates of Chlorella–Fungus Mixed Biomass into Poly(hydroxyalkanoates). Catalysts 2023, 13, 118. https://doi.org/10.3390/catal13010118
Senko O, Stepanov N, Maslova O, Efremenko E. Transformation of Enzymatic Hydrolysates of Chlorella–Fungus Mixed Biomass into Poly(hydroxyalkanoates). Catalysts. 2023; 13(1):118. https://doi.org/10.3390/catal13010118
Chicago/Turabian StyleSenko, Olga, Nikolay Stepanov, Olga Maslova, and Elena Efremenko. 2023. "Transformation of Enzymatic Hydrolysates of Chlorella–Fungus Mixed Biomass into Poly(hydroxyalkanoates)" Catalysts 13, no. 1: 118. https://doi.org/10.3390/catal13010118
APA StyleSenko, O., Stepanov, N., Maslova, O., & Efremenko, E. (2023). Transformation of Enzymatic Hydrolysates of Chlorella–Fungus Mixed Biomass into Poly(hydroxyalkanoates). Catalysts, 13(1), 118. https://doi.org/10.3390/catal13010118