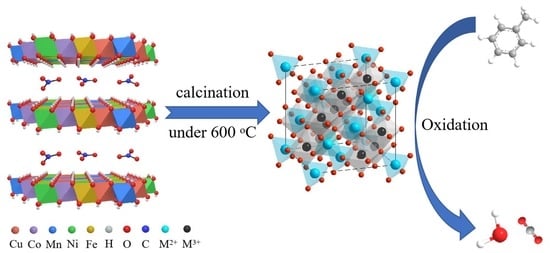
Development of Quinary Layered Double Hydroxide-Derived High-Entropy Oxides for Toluene Catalytic Removal
Abstract
:1. Introduction
2. Results and Discussion
Structure and Morphology of Catalysts
3. Materials and Methods
3.1. Chemicals
3.2. Catalysts Synthesis
3.3. Characterization
3.4. Catalytic Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Finlayson-Pitts, B.J.; James, N.; Pitts, J. Tropospheric air pollution: Ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science 1997, 276, 1045–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, L.; Ma, T.; Gao, Z.; Gao, J.; Wang, Z.; Xue, L.; Liu, H.; Liu, J. Characteristics and sources of volatile organic compounds during high ozone episodes: A case study at a site in the eastern Guanzhong Plain, China. Chemosphere 2020, 265, 129072. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.W.; Lou, J.C.; Lin, Y.C. Treatment of VOCs with molecular sieve catalysts in regenerative catalytic oxidizer. J. Hazard. Mater. 2010, 183, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Shahbazian-Yassar, R. Recent progress of high-entropy materials for energy storage and conversion. J. Mater. Chem. A 2021, 9, 782–823. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Dai, S. High-entropy materials for catalysis: A new frontier. Sci. Adv. 2021, 7, 1600. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, T.; Ye, Y.; Dai, W.; Zhu, Y.A.; Pan, Y. Stabilizing oxygen vacancy in entropy-engineered CoFe2O4-type catalysts for Co-prosperity of efficiency and stability in an oxygen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 32548–32555. [Google Scholar] [CrossRef]
- Hao, C.; Jie, F.; Zhang, P.; Peng, H.; Dai, S. Entropy–stabilized metal oxide solid solutions as CO oxidation catalysts with high–temperature stability. J. Mater. Chem. A 2018, 6, 11129–11133. [Google Scholar]
- Xu, H.; Zhang, Z.; Liu, J.; Do-Thanh, C.L.; Dai, S. Entropy-stabilized single-atom Pd catalysts via high-entropy fluorite oxide supports. Nat. Commun. 2020, 11, 3908. [Google Scholar] [CrossRef] [PubMed]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Accardo, G.; Frattini, D.; Dell’Agli, G. Entropy-stabilized oxides owning fluorite structure obtained by hydrothermal treatment. Materials 2020, 13, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, M.S.; Sundara, R. High entropy oxides-A cost-effective catalyst for the growth of high yield carbon nanotubes and their energy applications. ACS Appl. Mater. Interfaces 2019, 11, 30849–30857. [Google Scholar] [CrossRef] [PubMed]
- Stygar, M.; Dbrowa, J.; Modzierz, M.; Zajusz, M.; Danielewski, M. Formation and properties of high entropy oxides in Co-Cr-Fe-Mg-Mn-Ni-O system: Novel (Cr,Fe,Mg,Mn,Ni)3O4 and (Co,Cr,Fe,Mg,Mn)3O4 high entropy spinels. J. Eur. Ceram. Soc. 2019, 40, 1644–1650. [Google Scholar] [CrossRef]
- Zbigniew, G.; Grzegorz, S.; Maria, M.; Mirosław, S.; Juliusz, D.; Marek, Z.; Konrad, Ś.; Marek, D. Defect structure and transport properties of (Co,Cr,Fe,Mn,Ni)3O4 spinel-structured high entropy oxide. J. Eur. Ceram. Soc. 2020, 40, 835–839. [Google Scholar]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, B.; Hu, Z.; Zhen, M.; Guo, S.; Dong, F. Uncovering the synergy between Mn substitution and O vacancy in ZnAl-LDH photocatalyst for efficient toluene removal. Appl. Catal. B Environ. 2021, 296, 120376. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Wu, X.; Chen, Y. Recent advance on VOCs oxidation over layered double hydroxides derived mixed metal oxides. Chin. J. Catal. 2020, 41, 550–560. [Google Scholar] [CrossRef]
- Deng, W.; Tang, Q.; Huang, S.; Zhang, L.; Guo, L. Low temperature catalytic combustion of chlorobenzene over cobalt based mixed oxides derived from layered double hydroxides. Appl. Catal. B Environ. 2020, 278, 119336. [Google Scholar] [CrossRef]
- Huang, W.; Wang, H.; Zhu, X.; Yang, D.; Song, X. Highly efficient application of Mg/Al layered double oxides catalysts in the methanolysis of polycarbonate. Appl. Clay Sci. 2021, 202, 105986. [Google Scholar] [CrossRef]
- Grover, A.; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kim, K.-H. Zn-Al layered double hydroxides intercalated with surfactant: Synthesis and applications for efficient removal of organic dyes. J. Clean. Prod. 2019, 240, 118090. [Google Scholar] [CrossRef]
- Dabrowa, J.; Stygar, M.; Mikula, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2017, 216, 32–36. [Google Scholar] [CrossRef]
- Liang, B.; Ai, Y.; Wang, Y.; Liu, C.; Ouyang, S.; Liu, M. Spinel-type (FeCoCrMnZn)3O4 high-entropy oxide: Facile preparation and supercapacitor performance. Materials 2020, 13, 5798. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Wu, L.; Zhao, L.; Zheng, X.; Zhang, P. Entropy-driven chemistry reveals highly stable denary MgAl2O4-type catalysts. Chem Catalysis 2021, 1, 648–662. [Google Scholar] [CrossRef]
- Duan, C.; Li, X.; Wang, D.; Wang, Z.; Sun, H.; Zheng, R.; Liu, Y. Nanosized high entropy spinel oxide (FeCoNiCrMn)3O4 as a highly active and ultra-stable electrocatalyst for the oxygen evolution reaction. Sustain. Energy Fuels 2022, 6, 1479–1488. [Google Scholar] [CrossRef]
- Song, J.; Wang, S.; Xu, Y.; Liu, Q.; Zhao, Y. LDH derived MgAl2O4 spinel supported Pd catalyst for the low-temperature methane combustion: Roles of interaction between spinel and PdO. Appl. Catal. A Gen. 2021, 621, 118211. [Google Scholar] [CrossRef]
- Sun, G.; Sun, L.; Wen, H.; Jia, Z.; Huang, K.; Hu, C. From layered double hydroxide to spinel nanostructures: Facile synthesis and characterization of nanoplatelets and nanorods. J. Phys. Chem. B 2006, 110, 13375–13380. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Zhang, S.; Yuan, W.; Fang, X.; Yu, Q.; Qiu, X. Remediation of chromium-contaminated soil using calcined layered double hydroxides containing different divalent metals: Temperatures and mechanism. Chem. Eng. J. 2021, 425, 131405. [Google Scholar] [CrossRef]
- Feng, L.; Liu, X.; Yang, Q.; Liu, J.; Evans, D.G.; Duan, X. Synthesis and characterization of Ni1-x ZnxFe2O4 spinel ferrites from tailored layered double hydroxide precursors. Mater. Res. Bull. 2005, 40, 1244–1255. [Google Scholar]
- Zhao, J.; Bao, J.; Yang, S.; Niu, Q.; Xie, R.; Zhang, Q.; Chen, M.; Zhang, P.; Dai, S. Exsolution–dissolution of supported metals on high-entropy Co3MnNiCuZnOx: Toward sintering-resistant catalysis. ACS Catal. 2021, 11, 12247–12257. [Google Scholar] [CrossRef]
- František, K.; Tomáš, G.; Dorničák, V.t. Thermal behaviour of Ni–Mn layered double hydroxide and characterization of formed oxides. Solid State Sci. 2003, 5, 1019–1026. [Google Scholar]
- Bertolini, G.R.; Cecilia, J.A.; Mairelestorres, P. Gas-phase hydrogenation of furfural to furfuryl alcohol over Cu-ZnO-Al2O3 catalysts prepared from layered double hydroxides. Catalysts 2020, 10, 486. [Google Scholar] [CrossRef]
- Sonia, S.; Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Hydrothermal synthesis of highly stable CuO nanostructures for efficient photocatalytic degradation of organic dyes. Mater. Sci. Semicond. Process. 2015, 30, 585–591. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik-Zym, A.; Dula, R.; Bielańska, E.; Rojek, W.; Machej, T.; Socha, R.P.; Lityńska-Dobrzyńska, L.; Bahranowski, K.; Serwicka, E.M. Composites derived from exfoliated Laponite and Mn-Al hydrotalcite prepared in inverse microemulsion: A new strategy for design of robust VOCs combustion catalysts. Appl. Catal. B Environ. 2017, 211, 46–56. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Liu, W.; Xue, T.; Liu, C.; Zhang, Y.; Liu, L.; Wang, Q.; Qi, F.; Xu, B. Novel CuCo2O4 composite spinel with a meso-macroporous nanosheet structure for sulfate radical formation and benzophenone-4 degradation: Interface reaction, degradation pathway, and DFT calculation. ACS Appl. Mater. Interfaces 2020, 12, 20522–20535. [Google Scholar] [CrossRef]
- Qi, Z.; Mo, S.; Chen, B.; Zhang, W.; Huang, C.; Ye, D. Hierarchical Co3O4 nanostructures in-situ grown on 3D nickel foam towards toluene oxidation. Mol. Catal. 2018, 454, 12–20. [Google Scholar]
- Ren, Q.; Feng, Z.; Mo, S.; Huang, C.; Li, S.; Zhang, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. 1D-Co3O4, 2D-Co3O4, 3D-Co3O4 for catalytic oxidation of toluene. Catal. Today 2019, 332, 160–167. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Zuo, J.; Feng, X.; Wang, X.; Zhang, T.; Zhang, K.; Jiang, L. Insights into the high performance of Mn-Co oxides derived from metal-organic frameworks for total toluene oxidation. J. Hazard. Mater. 2018, 349, 119–127. [Google Scholar] [CrossRef]
- Ning, F.; Shao, M.; Zhang, C.; Xu, S.; Wei, M.; Duan, X. Co3O4@layered double hydroxide core/shell hierarchical nanowire arrays for enhanced supercapacitance performance. Nano Energy 2014, 7, 134–142. [Google Scholar] [CrossRef]
- Cai, S.; Liu, J.; Zha, K.; Li, H.; Shi, L.; Zhang, D. A general strategy for the in situ decoration of porous Mn–Co bi-metal oxides on metal mesh/foam for high performance de-NOx monolith catalysts. Nanoscale 2017, 9, 5648–5657. [Google Scholar] [CrossRef]
- Shen, Z.; Hu, F.Y.; Li, J. Hierarchical core–shell Al2O3@Pd-CoAlO microspheres for low-temperature toluene combustion. ACS Catal. 2016, 6, 3433–3441. [Google Scholar]
- Jiang, S.; Handberg, E.S.; Liu, F.; Liao, Y.; Wang, H.; Li, Z.; Song, S. Effect of doping the nitrogen into carbon nanotubes on the activity of NiO catalysts for the oxidation removal of toluene. Appl. Catal. B Environ. 2014, 160–161, 716–721. [Google Scholar] [CrossRef]
- Khadija, T.K.; Rehana, S.; Bebi, P.; Atif, J.; Shamaila, S.; Ayesha, S.; Sofia, S.; Ghulam, A. Hydrothermal synthesis of neodymium oxide nanoparticles and its nanocomposites with manganese oxide as electrode materials for supercapacitor application. J. Alloys Compd. 2020, 815, 152104. [Google Scholar]
- Liu, X.; Du, Y.; Zou, C.; Liu, L.; Yang, B.; Wu, X. NH3-SCR performance enhancement of LDHs-based NiMnFe-mixed oxides by two-phase coexistence and cooperation. ChemistrySelect 2019, 4, 9488–9496. [Google Scholar] [CrossRef]
- Monica, R.; Géraldine, L.; Florica, P.; Cătălin, N.; Didier, T.; Ioan-Cezar, M. Influence of Mn content on the catalytic properties of Cu-(Mn)-Zn-Mg-Al mixed oxides derived from LDH precursors in the total oxidation of methane. Catal. Today 2017, 306, 276–286. [Google Scholar]
- Wen, Y.; Zhao, S.; Yi, H.; Gao, F.; Yu, Q.; Liu, J.; Tang, T.; Tang, X. Efficient catalytic oxidation of methyl mercaptan to sulfur dioxide with NiCuFe mixed metal oxides. Environ. Technol. Innov. 2022, 26, 102252. [Google Scholar] [CrossRef]
- Siham, B.; Noel-Andrés, G.-M.; Miguel-Ángel, G.-G.; Dariusz, Ś.; Françoise, Q.; Nathalie, T. Study and modelling of kinetics of the oxidation of VOC catalyzed by nanosized Cu–Mn spinels prepared via an alginate route. Appl. Catal. A Gen. 2015, 504, 203–210. [Google Scholar]





| Samples | BET Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| HEO-400 | 140.0 | 38.6 | 1.35 |
| HEO-500 | 79.6 | 27.8 | 0.55 |
| HEO-600 | 49.2 | 23.9 | 0.29 |
| HEO-700 | 16.7 | 23.4 | 0.12 |
| HEO-800 | 9.4 | 22.2 | 0.05 |
| Samples | Cu2+/(Cu+ + Cu2+) | Co3+/(Co2+ + Co3+) | Mn4+/(Mn2+ + Mn3+ + Mn4+) | Ni3+/(Ni2+ + Ni3+) | Fe3+/(Fe2+ + Fe3+) | Oβ/(Oα + Oβ) |
|---|---|---|---|---|---|---|
| HEO-400 | ~1 | 0.392 | 0.262 | 0.584 | 0.707 | 0.413 |
| HEO-500 | 0.962 | 0.407 | 0.293 | 0.566 | 0.694 | 0.421 |
| HEO-600 | 0.950 | 0.432 | 0.306 | 0.532 | 0.682 | 0.442 |
| HEO-700 | 0.944 | 0.444 | 0.396 | 0.524 | 0.671 | 0.454 |
| HEO-800 | 0.933 | 0.449 | 0.454 | 0.515 | 0.671 | 0.478 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, T.; Wang, Y.; Yang, L.; Li, Z.; Gao, Y.; Wang, Q. Development of Quinary Layered Double Hydroxide-Derived High-Entropy Oxides for Toluene Catalytic Removal. Catalysts 2023, 13, 119. https://doi.org/10.3390/catal13010119
Xue T, Wang Y, Yang L, Li Z, Gao Y, Wang Q. Development of Quinary Layered Double Hydroxide-Derived High-Entropy Oxides for Toluene Catalytic Removal. Catalysts. 2023; 13(1):119. https://doi.org/10.3390/catal13010119
Chicago/Turabian StyleXue, Tianshan, Yiping Wang, Li Yang, Zhe Li, Yanshan Gao, and Qiang Wang. 2023. "Development of Quinary Layered Double Hydroxide-Derived High-Entropy Oxides for Toluene Catalytic Removal" Catalysts 13, no. 1: 119. https://doi.org/10.3390/catal13010119
APA StyleXue, T., Wang, Y., Yang, L., Li, Z., Gao, Y., & Wang, Q. (2023). Development of Quinary Layered Double Hydroxide-Derived High-Entropy Oxides for Toluene Catalytic Removal. Catalysts, 13(1), 119. https://doi.org/10.3390/catal13010119