Enhanced Oil Recovery by In-Reservoir Hydrogenation of Carbon Dioxide Using Na-Fe3O4
Abstract
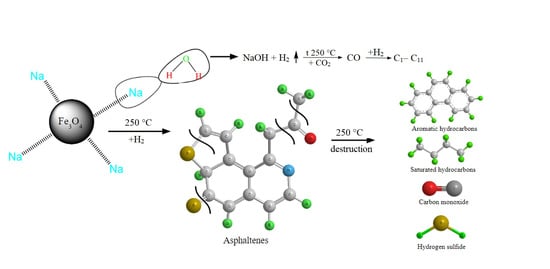
:1. Introduction
2. Results and Discussions
2.1. Characterization of the Catalyst
2.1.1. FT-IR Analysis of the Achieved Catalysts
2.1.2. XRD
2.1.3. SEM and EDX
2.2. Upgrading Performance of the Synthesized Catalyst Nanoparticles
2.2.1. Gas Composition of the Products
2.2.2. Group Composition of Crude Oil Samples
2.2.3. Viscosity of Heavy Oil before and after CO2 Hydrogenation
2.2.4. Elemental Composition of Oil Samples
2.2.5. Structural Characterization of Oil Samples Using FT-IR and EPR Spectra before and after Upgrading of Heavy Oil
2.2.6. The GC-MS Results of Saturated and Aromatics Fractions of Oil Samples
3. Materials and Methods
3.1. Synthesis of Na-Fe3O4 Nanoparticles
3.2. Characterization of the Obtained Catalyst
3.2.1. X-ray Diffraction (XRD) Analysis
3.2.2. Scanning Electron Microscope (SEM) and Energy-Dispersive X-ray Spectrometer (EDX) Analysis
3.3. The Activity of the Synthesized Catalyst Nanoparticles
3.3.1. Heavy Oil Catalytic Upgrading Experiments
3.3.2. Gas Chromatography (GC) of the Evolved Gasses
3.3.3. SARA-Analysis
3.3.4. Elemental Analysis
3.3.5. Fourier Transform Infrared (FT-IR) Spectral Analysis
3.3.6. Viscosity Measurements
3.3.7. EPR Spectral Analysis
3.3.8. Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis
4. Conclusions
- Increasing the content of aromatics from 32.5 wt.% to 49 wt.% and reducing the content of resins from 32 wt.% to 18 wt.%, indicating the destructive hydrogenation of high-molecular compounds in the composition of crude oil.
- Reducing the viscosity of heavy oil samples from 3250 mPa.s to 2250 mPa.s and increasing H/C from 1.32 to 1.74.
- Increasing the relative intensity of free, stable radicals in the oil structure by 75%.
- Increase in the content of low-molecular n-alkanes (C14 –C17) and relative content of C2–C8 gaseous products from 3.76 to 4.39 wt.%.
- Increase in the relative content of alkyl benzenes in aromatics fraction after CO2-assisted hydrothermal treatment of oil from 13% to 38%
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, T.; Jang, S.-H.; Jung, S.; Kim, S.; Park, Y.-K.; Moon, D.H.; Kwon, E.E. CO2 effects on catalytic pyrolysis of yard trimming over concrete waste. Chem. Eng. J. 2020, 396, 125331. [Google Scholar] [CrossRef]
- Bhowmik, D. Global Carbon di Oxide Emissions in Hamilton Filter Model. Int. J. Environ. Agric. Biotechnol. 2020, 5, 1250–1258. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, E.; Tang, J. Insight on Reaction Pathways of Photocatalytic CO2 Conversion. ACS Catal. 2022, 12, 7300–7316. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Kothandaraman, J.; Goeppert, A.; Prakash, G.S. Advances in catalytic homogeneous hydrogenation of carbon dioxide to methanol. J. CO2 Util. 2018, 23, 212–218. [Google Scholar] [CrossRef]
- Kangvansura, P.; Chew, L.M.; Saengsui, W.; Santawaja, P.; Poo-Arporn, Y.; Muhler, M.; Schulz, H.; Worayingyong, A. Product distribution of CO2 hydrogenation by K- and Mn-promoted Fe catalysts supported on N -functionalized carbon nanotubes. Catal. Today 2016, 275, 59–65. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Catalytic thermal conversion of CO2 into fuels: Perspective and challenges. Renew. Sustain. Energy Rev. 2019, 115, 109333. [Google Scholar] [CrossRef]
- Quitian, A.; Fernández, Y.; Ancheyta, J. Viscosity Reduction of Heavy Oil during Slurry-Phase Hydrocracking. Chem. Eng. Technol. 2019, 42, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Alotaibi, F.M.; González-Cortés, S.; Alotibi, M.F.; Xiao, T.; Al-Megren, H.; Yang, G.; Edwards, P.P. Enhancing the production of light olefins from heavy crude oils: Turning challenges into opportunities. Catal. Today 2018, 317, 86–98. [Google Scholar] [CrossRef]
- Shafiei, A.; Dusseault, M.B. Geomechanics of thermal viscous oil production in sandstones. J. Pet. Sci. Eng. 2013, 103, 121–139. [Google Scholar] [CrossRef]
- Butler, R. Some Recent Developments in SAGD. J. Can. Pet. Technol. 2001, 40, 18–22. [Google Scholar] [CrossRef]
- Muradov, N. How to produce hydrogen from fossil fuels without CO2 emission. Int. J. Hydrogen Energy 1993, 18, 211–215. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, H. The Effect of Hydrogen Donor Additive on the Viscosity of Heavy Oil during Steam Stimulation. Energy Fuels 2002, 16, 842–846. [Google Scholar] [CrossRef]
- Aliev, F.; Mirzaev, O.; Kholmurodov, T.; Slavkina, O.; Vakhin, A. Utilization of Carbon Dioxide via Catalytic Hydrogenation Processes during Steam-Based Enhanced Oil Recovery. Processes 2022, 10, 2306. [Google Scholar] [CrossRef]
- Pratama, R.A.; Babadagli, T. A review of the mechanics of heavy-oil recovery by steam injection with chemical additives. J. Pet. Sci. Eng. 2022, 208, 109717. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Shen, D.; Shi, L.; Zhang, Z.; Sun, X.; Jiang, Q. CO2 huff-n-puff process to enhance heavy oil recovery and CO2 storage: An integration study. Energy 2022, 239, 122003. [Google Scholar] [CrossRef]
- Orr Jr, F.M.; Taber, J.J. Use of Carbon Dioxide in Enhanced Oil Recovery. Science 1984, 224, 563–569. [Google Scholar] [CrossRef]
- Jha, K.N. A Laboratory Study of Heavy Oil Recovery with Carbon Dioxide. J. Can. Pet. Technol. 1986, 25, 54–63. [Google Scholar] [CrossRef]
- Vogt, C.; Groeneveld, E.; Kamsma, G.; Nachtegaal, M.; Lu, L.; Kiely, C.J.; Berben, P.H.; Meirer, F.; Weckhuysen, B.M. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018, 1, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Minh, D.P.; Roger, A.-C.; Parkhomenko, K.; L’Hospital, V.; de Vasconcelos, B.R.; Ro, K.; Mahajan, D.; Chen, L.; Singh, S.; Vo, D.-V.N. Selective Hydrogenation of Carbon Dioxide into Methanol. In Conversion of Carbon Dioxide into Hydrocarbons Vol. 2 Technology; Springer: Cham, Switzerland, 2020; pp. 111–157. [Google Scholar]
- Li, Z.; Qu, Y.; Wang, J.; Liu, H.; Li, M.; Miao, S.; Li, C. Highly Selective Conversion of Carbon Dioxide to Aromatics over Tandem Catalysts. Joule 2019, 3, 570–583. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Jung, K.-D.; Yoon, S. Hydrogenation of CO2 to Formate Using a Simple, Recyclable, and Efficient Heterogeneous Catalyst. Inorg. Chem. 2019, 58, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yi, Y.; Wang, L.; Guo, H.; Bogaerts, A. Hydrogenation of Carbon Dioxide to Value-Added Chemicals by Heterogeneous Catalysis and Plasma Catalysis. Catalysts 2019, 9, 275. [Google Scholar] [CrossRef] [Green Version]
- Guil-López, R.; Mota, N.; Llorente, J.; Millán, E.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials 2019, 12, 3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.K.; Butolia, P.; Jo, H.; Irshad, M.; Han, D.; Nam, K.-W.; Kim, J. Selective Conversion of Carbon Dioxide into Liquid Hydrocarbons and Long-Chain α-Olefins over Fe-Amorphous AlO x Bifunctional Catalysts. ACS Catal. 2020, 10, 10325–10338. [Google Scholar] [CrossRef]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Usui, M.; Takezawa, N. Mechanism of the Reverse Water Gas Shift Reaction over Cu/ZnO Catalyst. J. Catal. 1992, 134, 220–225. [Google Scholar] [CrossRef]
- Zhu, M.; Wachs, I.E. Iron-Based Catalysts for the High-Temperature Water–Gas Shift (HT-WGS) Reaction: A Review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Kung, H.H. Deactivation of methanol synthesis catalysts—A review. Catal. Today 1992, 11, 443–453. [Google Scholar] [CrossRef]
- Lunde, P.J.; Kester, F.L. Carbon Dioxide Methanation on a Ruthenium Catalyst. Ind. Eng. Chem. Process Des. Dev. 1974, 13, 27–33. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Takht Ravanchi, M. Carbon dioxide utilization for methane production: A thermodynamic analysis. J. Pet. Sci. Eng. 2015, 134, 14–22. [Google Scholar] [CrossRef]
- Koschany, F.; Schlereth, D.; Hinrichsen, O. On the Kinetics of the Methanation of Carbon Dioxide on Coprecipitated NiAl (O) x. Appl. Catal. B 2016, 181, 504–516. [Google Scholar] [CrossRef]
- Cai, M.; Wen, J.; Chu, W.; Cheng, X.; Li, Z. Methanation of carbon dioxide on Ni/ZrO2-Al2O3 catalysts: Effects of ZrO2 promoter and preparation method of novel ZrO2-Al2O3 carrier. J. Nat. Gas Chem. 2011, 20, 318–324. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2020, 11, 28. [Google Scholar] [CrossRef]
- Tada, S.; Ochieng, O.J.; Kikuchi, R.; Haneda, T.; Kameyama, H. Promotion of CO2 Methanation Activity and CH4 Selectivity at Low Temperatures over Ru/CeO2/Al2O3 Catalysts. Int. J. Hydrog. Energy 2014, 39, 10090–10100. [Google Scholar] [CrossRef]
- Nakayama, T.; Ichikuni, N.; Sato, S.; Nozaki, F. Ni/Mgo catalyst prepared using citric acid for hydrogenation of carbon dioxide. Appl. Catal. A Gen. 1997, 158, 185–199. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G.L. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pretreatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379. [Google Scholar] [CrossRef]
- Wang, W.; Gong, J. Methanation of carbon dioxide: An overview. Front. Chem. Sci. Eng. 2011, 5, 2–10. [Google Scholar] [CrossRef]
- De Smit, E.; Weckhuysen, B.M. The Renaissance of Iron-Based Fischer–Tropsch Synthesis: On the Multifaceted Catalyst Deactivation Behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef]
- Evdokimenko, N.D.; Kapustin, G.I.; Tkachenko, O.P.; Kalmykov, K.B.; Kustov, A.L. Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons. Molecules 2022, 27, 1065. [Google Scholar] [CrossRef]
- Prasad, P.S.S.; Bae, J.W.; Jun, K.-W.; Lee, K.-W. Fischer–Tropsch Synthesis by Carbon Dioxide Hydrogenation on Fe-Based Catalysts. Catal. Surv. Asia 2008, 12, 170–183. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ge, Q.; Tsubaki, N. Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons. J. Mater. Chem. A 2018, 6, 23244–23262. [Google Scholar] [CrossRef]
- Petala, A.; Panagiotopoulou, P. Methanation of CO2 over alkali-promoted Ru/TiO2 catalysts: I. Effect of alkali additives on catalytic activity and selectivity. Appl. Catal. B Environ. 2018, 224, 919–927. [Google Scholar] [CrossRef]
- Herranz, T.; Rojas, S.; Perez-Alonso, F.J.; Ojeda, M.; Terreros, P.; Fierro, J. Hydrogenation of carbon oxides over promoted Fe-Mn catalysts prepared by the microemulsion methodology. Appl. Catal. A Gen. 2006, 311, 66–75. [Google Scholar] [CrossRef]
- Bukur, D.B.; Mukesh, D.; Patel, S.A. Promoter effects on precipitated iron catalysts for Fischer-Tropsch synthesis. Ind. Eng. Chem. Res. 1990, 29, 194–204. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-Liquid via Synthesis of Methanol, DME or Fischer–Tropsch-Fuels: A Review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, B.; Zhu, A.; Gong, W.; Liu, C. The Simultaneous Activation of Methane and Carbon Dioxide to C2 Hydrocarbons under Pulse Corona Plasma over La2O3/γ-Al2O3 Catalyst. Catal. Today 2002, 72, 223–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Jacobs, G.; Sparks, D.E.; Dry, M.E.; Davis, B.H. CO and CO2 hydrogenation study on supported cobalt Fischer–Tropsch synthesis catalysts. Catal. Today 2002, 71, 411–418. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Choi, Y.H.; Ra, E.C.; Kim, E.H.; Kim, K.Y.; Jang, Y.J.; Kang, K.-N.; Choi, S.H.; Jang, J.-H.; Lee, J.S. Sodium-Containing Spinel Zinc Ferrite as a Catalyst Precursor for the Selective Synthesis of Liquid Hydrocarbon Fuels. ChemSusChem 2017, 10, 4764–4770. [Google Scholar] [CrossRef]
- Wen, C.; Jiang, J.; Chiliu, C.; Tian, Z.; Xu, X.; Wu, J.; Wang, C.; Ma, L. Single-Step Selective Conversion of Carbon Dioxide to Aromatics over Na-Fe3O4/Hierarchical HZSM-5 Zeolite Catalyst. Energy Fuels 2020, 34, 11282–11289. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly Converting CO2 into a Gasoline Fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakhin, A.V.; Khelkhal, M.A.; Mukhamatdinov, I.I.; Mukhamatdinova, R.E.; Tajik, A.; Slavkina, O.V.; Malaniy, S.Y.; Gafurov, M.R.; Nasybullin, A.R.; Morozov, O.G. Changes in Heavy Oil Saturates and Aromatics in the Presence of Microwave Radiation and Iron-Based Nanoparticles. Catalysts 2022, 12, 514. [Google Scholar] [CrossRef]
- Kholmurodov, T.; Aliev, F.; Mirzaev, O.; Dengaev, A.; Tajik, A.; Vakhin, A. Hydrothermal In-Reservoir Upgrading of Heavy Oil in the Presence of Non-Ionic Surfactants. Processes 2022, 10, 2176. [Google Scholar] [CrossRef]
- Kim, C.; Yoo, C.-J.; Oh, H.-S.; Min, B.K.; Lee, U. Review of carbon dioxide utilization technologies and their potential for industrial application. J. CO2 Util. 2022, 65, 102239. [Google Scholar] [CrossRef]
- Gafurov, M.; Volodin, M.; Rodionov, A.; Sorokina, A.; Dolomatov, M.; Petrov, A.; Vakhin, A.; Mamin, G.; Orlinskii, S. EPR study of spectra transformations of the intrinsic vanadyl-porphyrin complexes in heavy crude oils with temperature to probe the asphaltenes’ aggregation. J. Pet. Sci. Eng. 2018, 166, 363–368. [Google Scholar] [CrossRef]














| Elements | Relative Content (wt.%) | Relative Content (Atom.%) |
|---|---|---|
| O | 4.82 | 16.11 |
| Na | 10.63 | 21.44 |
| Al | 0.17 | 0.26 |
| Si | 0.12 | 0.17 |
| Cr | 0.16 | 0.13 |
| Mn | 0.32 | 0.25 |
| Fe | 83.78 | 61.64 |
| Sum | 100 | 100 |
| Gas Composition, wt.% | Samples | |
|---|---|---|
| HTT | HTT + Na-Fe3O4 | |
| CH4 | 0.96 | 0.42 |
| C2-C8 | 3.76 | 4.39 |
| H2 | 1.71 | 0.39 |
| O2 | 5.45 | 1.74 |
| N2 | 31.20 | 39.28 |
| H2S | 13.78 | 16.05 |
| Other gasses | 43.16 | 37.73 |
| Total | 100 | 100 |
| Sample | Elemental Composition, % | |||||
|---|---|---|---|---|---|---|
| C | H | N | S | O | H/C | |
| Initial crude oil | 79.01 | 8.74 | 0.45 | 4.85 | 5.85 | 1.32 |
| HTT | 82.11 | 11.30 | 0.00 | 4.71 | 1.88 | 1.64 |
| HTT + catalyst | 82.42 | 12.01 | 0.03 | 4.60 | 0.94 | 1.74 |
| Properties | Coefficients | Samples | ||
|---|---|---|---|---|
| Initial oil | HTT | HTT + Catalyst | ||
| Aromaticity | C1 | 0.86 | 0.88 | 0.84 |
| Branching | C3 | 0.98 | 0.96 | 0.98 |
| Aliphaticity | C4 | 2.52 | 2.44 | 2.52 |
| Sulfurization | C5 | 0.86 | 0.89 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliev, F.; Kholmurodov, T.; Mirzayev, O.; Tajik, A.; Mukhamadiev, N.; Slavkina, O.; Nourgalieva, N.; Vakhin, A. Enhanced Oil Recovery by In-Reservoir Hydrogenation of Carbon Dioxide Using Na-Fe3O4. Catalysts 2023, 13, 153. https://doi.org/10.3390/catal13010153
Aliev F, Kholmurodov T, Mirzayev O, Tajik A, Mukhamadiev N, Slavkina O, Nourgalieva N, Vakhin A. Enhanced Oil Recovery by In-Reservoir Hydrogenation of Carbon Dioxide Using Na-Fe3O4. Catalysts. 2023; 13(1):153. https://doi.org/10.3390/catal13010153
Chicago/Turabian StyleAliev, Firdavs, Temurali Kholmurodov, Oybek Mirzayev, Arash Tajik, Nurali Mukhamadiev, Olga Slavkina, Nuriya Nourgalieva, and Alexey Vakhin. 2023. "Enhanced Oil Recovery by In-Reservoir Hydrogenation of Carbon Dioxide Using Na-Fe3O4" Catalysts 13, no. 1: 153. https://doi.org/10.3390/catal13010153
APA StyleAliev, F., Kholmurodov, T., Mirzayev, O., Tajik, A., Mukhamadiev, N., Slavkina, O., Nourgalieva, N., & Vakhin, A. (2023). Enhanced Oil Recovery by In-Reservoir Hydrogenation of Carbon Dioxide Using Na-Fe3O4. Catalysts, 13(1), 153. https://doi.org/10.3390/catal13010153