Application of Endoxylanases of Bacillus halodurans for Producing Xylooligosaccharides from Empty Fruit Bunch
Abstract
:1. Introduction
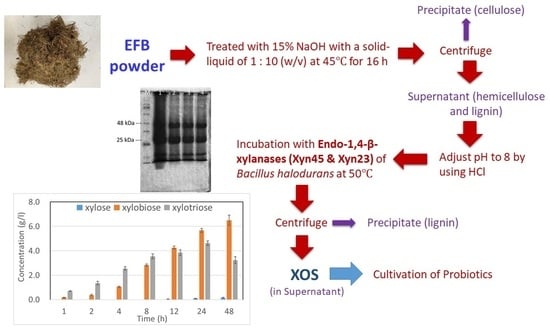
2. Results
2.1. Production of Endo-1,4-β-Xylanases
2.2. XOS Production
2.3. Promotion of the Growth and Metabolism of Bifidobacteria and Lactobacillus
3. Discussion
4. Materials and Methods
4.1. Preparation of Nonrecombinant Endoxylanases
4.2. Preparation of Recombinant Endoxylanase Xyn45
4.3. Analysis of Enzyme Activity and Protein Assay
4.4. XOS Production from EFB through Enzymatic Reaction
4.5. Fermentation of Probiotics on XOS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takami, H.; Nakasone, K.; Takagi, Y.; Maeno, G.; Sasaki, R.; Masui, N.; Fuji, F.; Hirama, C.; Nakamura, Y.; Ogasawara, N.; et al. Complete genome sequence of the alkali-philic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res. 2000, 28, 4317–4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Ratanakhanokchai, K.; Piyatheerawong, W.; Kyu, K.L.; Rho, M.S.; Kim, Y.S.; Om, A.; Lee, J.W.; Jhee, O.H.; Chon, G.H.; et al. Production and location of xylanolytic enzymes in alkaliphilic Bacillus sp. K-1. J. Microbiol. Biotechnol. 2006, 16, 921–926. [Google Scholar]
- Honda, Y.; Kitaoka, M. A family 8 glycoside hydrolase from Bacillus halodurans C-125 (BH2105) is a reducing end xylose-releasing exo-oligoxylanase. J. Biol. Chem. 2004, 279, 55097–55103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.S.; Tseng, M.J.; Lee, W.C. Production of xylooligosaccharides using immobilized endo-xylanase of Bacillus halodurans. Process Biochem. 2011, 46, 2117–2121. [Google Scholar] [CrossRef]
- Polizeli, M.L.; Rizzatti, A.C.; Monti, R.; Terenzi, H.F.; Jorge, J.A.; Amorim, D.S. Xylanases from fungi: Properties and industrial applications. Appl. Microbiol. Biotechnol. 2005, 67, 577–591. [Google Scholar] [CrossRef]
- Tseng, M.J.; Yap, M.N.; Ratanakhanokchai, K.; Kyu, K.L.; Chen, S.T. Purification and characterization of two cellulase free xylanases from an alkaliphilic Bacillus firmus. Enzyme Microb. Technol. 2002, 30, 590–595. [Google Scholar] [CrossRef]
- Chang, P.; Tsai, W.S.; Tsai, C.L.; Tseng, M.J. Cloning and characterization of two thermostable xylanases from an alkaliphilic Bacillus firmus. Biochem. Biophys. Res. Commun. 2004, 319, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Beg, Q.K.; Kapoor, M.; Mahajan, L.; Hoondal, G.S. Microbial xylanases and their industrial applications: A review. Appl. Microbiol. Biotechnol. 2001, 56, 326–338. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L. Enzymatic hydrolysis of pretreated biomass. In Technologies for Biochemical Conversion of Biomass; Elsevier: Amsterdam, The Netherlands, 2016; pp. 65–99. [Google Scholar]
- Bhardwaj, N.; Kumar, B.; Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- GeonomeNet Database: Bacillus halodurans C-125 DNA, Complete Genome. Available online: https://www.genome.jp/dbget-bin/www_bget?refseq+NC_002570 (accessed on 16 November 2022).
- Wamalwa, B.M.; Zhao, G.; Sakka, M.; Shiundu, P.M.; Kimura, T.; Sakka, K. High-level heterologous expression of Bacillus halodurans putative xylanase xyn11a (BH0899) in Kluyveromyces lactis. Biosci. Biotechnol. Biochem. 2007, 71, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Prakash, P.; Jayalakshmi, S.K.; Prakash, B.; Rubul, M.; Sreeramulu, K. Production of alkaliphilic, halotolerent, thermostable cellulase free xylanase by Bacillus halodurans PPKS-2 using agro waste: Single step purification and characterization. World J. Microbiol. Biotechnol. 2012, 28, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Charalampopoulos, D.; Rastall, R.A. Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Bioresour. Technol. 2014, 152, 526–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aachary, A.A.; Siddalingaiya, P. Xylooligosaccharides (XOS) as an emerging prebiotic: Microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Compr. Rev. Food Sci. Food Saf. 2010, 10, 2–16. [Google Scholar] [CrossRef]
- Han, J.; Kim, J. Process simulation and optimization of 10-MW EFB power plant. Comput. Aided Chem. Eng. 2018, 43, 723–729. [Google Scholar]
- Sudiyani, Y.; Styarini, D.; Triwahyuni, E.; Sudiyarmanto; Sembiring, K.C.; Aristiawan, Y.; Abimanyu, H.; Han, M.H. Utilization of biomass waste empty fruit bunch fiber of palm oil for bioethanol production using pilot—Scale unit. Energy Procedia 2013, 32, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Millati, R.; Wikandari, R.; Trihandayani, E.; Cahyanto, M.; Taherzadeh, M.; Niklasson, C. Ethanol from oil palm empty fruit bunch via dilute-acid hydrolysis and fermentation by Mucor indicus and Saccharomyces cerevisiae. Agric. J. 2011, 6, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.; Sulaiman, F.; Gerhauser, H. Characterisation of oil palm empty fruit bunches for fuel application. J. Phys. Sci. 2011, 22, 1–24. [Google Scholar]
- Baharuddin, A.S.; Yunos, N.S.H.; Mahmud, N.A.N.; Zakaria, R.; Yunos, K.F. Effect of high-pressure steam treatment on enzymatic saccharification of oil palm empty fruit bunches. BioResources 2012, 7, 3525–3538. [Google Scholar]
- Palamae, S.; Dechatiwongse, P.; Choorit, W.; Chisti, Y.; Prasertsan, P. Cellulose and hemicellulose recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carbohydr. Polym. 2017, 155, 491–497. [Google Scholar] [CrossRef]
- Noorshamsiana, A.W.; Faizah, J.N.; Kamarudin, H.; Eliyanti, A.O.N.; Fatiha, I.; Astimar, A.A. Integrated production of prebiotic xylooligosaccharides and high value cellulose from oil palm biomass. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022044. [Google Scholar] [CrossRef]
- Rathamat, Z.; Choorit, W.; Chisti, Y.; Prasertsan, P. Two-step isolation of hemicellulose from oil palm empty fruit bunch fibers and its use in production of xylooligosaccharide prebiotic. Ind. Crops Prod. 2021, 160, 113124. [Google Scholar] [CrossRef]
- Morelli, L.; Callegari, M.L.; Patrone, V. Prebiotics, Probiotics, and Synbiotics: A Bifidobacterial View; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Li, Z.; Summanen, P.H.; Komoriya, T.; Finegold, S.M. In vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp and Lactobacillus spp. Int. J. Food Sci. Nutr. 2015, 66, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.K.; Jayapal, N.; Jayaram, C.; Roy, S.; Kolte, A.P.; Senani, S.; Sridhar, M. Xylooligosaccharides as prebiotics from agricultural by-products: Production and applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 62–71. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Li, Z.; Yan, B.; Pei, W.; Wu, H. The preparation technology and application of xylo-oligosaccharide as prebiotics in different fields: A review. Front Nutr. 2022, 9, 996811. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Kudo, T.; Ikura, Y.; Horikoshi, K. Two types of xylanases of alkalophilic Bacillus sp. No. C-125. Can. J. Microbiol. 1985, 31, 538–542. [Google Scholar] [CrossRef]
- Cho, S.G.; Choi, Y.J. Catabolite repression of the xylanase gene (xynA) expression in Bacillus stearothermophilus no. 236 and B. subtilis. Biosci. Biotechnol. Biochem. 1999, 63, 2053–2058. [Google Scholar] [CrossRef] [Green Version]
- Shulami, S.; Shenker, O.; Langut, Y.; Lavid, N.; Gat, O.; Zaide, G.; Zehavi, A.; Sonenshein, A.L.; Shoham, Y. Multiple regulatory mechanisms control the expression of the Geobacillus stearothermophilus gene for extracellular xylanase. J. Biol. Chem. 2014, 289, 25957–25975. [Google Scholar] [CrossRef] [Green Version]
- Hueck, C.J.; Hillen, W. Catabolite repression in Bacillus subtilis: A global regulatory mechanism for the gram-positive bacteria? Mol. Microbiol. 1995, 15, 395–401. [Google Scholar] [CrossRef]
- Moreno, M.S.; Schneider, B.L.; Maile, R.; Weyler, W.; Saier, M.H., Jr. Catabolite repression mediated by the CcpA protein in Bacillus subtilis: Novel modes of regulation revealed by whole-genome analyses. Mol. Microbiol. 2001, 39, 1366–1381. [Google Scholar] [CrossRef]
- Fujita, Y. Carbon Catabolite Control of the Metabolic Network in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2009, 73, 245–259. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.D.; Schmalisch, M.H.; Stülke, J.; Görke, B. Carbon catabolite repression in Bacillus subtilis: Quantitative analysis of repression exerted by different carbon sources. J. Bacteriol. 2008, 190, 7275–7284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Cao, R.; Wang, M.; Lin, Q.; Zhan, R.; Xu, H.; Wang, S. A novel thermostable GH10 xylanase with activities on a wide variety of cellulosic substrates from a xylanolytic Bacillus strain exhibiting significant synergy with commercial Celluclast 1.5 L in pretreated corn stover hydrolysis. Biotechnol. Biofuels 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Satyanarayana, T. Biochemical and thermodynamic characteristics of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles 2013, 17, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Mamo, G.; Hatti-Kaul, R.; Mattiasson, B. A thermostable alkaline active endo-beta-1-4-xylanase from Bacillus halodurans S7: Purification and characterization. Enzyme Microb. Technol. 2006, 39, 1492–1498. [Google Scholar] [CrossRef]
- Faryar, R.; Linares-Pastén, J.A.; Immerzeel, P.; Mamo, G.; Andersson, M.; Stålbrand, H.; Mattiasson, B.; Karlsson, E.N. Production of prebiotic xylooligosaccharides from alkaline extracted wheat straw using the K80R-variant of a thermostable alkali-tolerant xylanase. Food Bioprod. Process. 2015, 93, 1–10. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wang, R.; Liu, J.; Zhang, Y.; Liu, L.; Wang, F.; Yuan, H. Cooperation of hydrolysis modes among xylanases reveals the mechanism of hemicellulose hydrolysis by Penicillium chrysogenum P33. Microb. Cell Fact. 2019, 18, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliev, I.; Vasileva, T.; Bivolarski, V.; Momchilova, A.; Ivanova, I. Metabolic profiling of xylooligosaccharides by Lactobacilli. Polymers 2020, 12, 2387. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Wang, C. In vitro fermentation of xylooligosaccharides from wheat bran insoluble dietary fiber by Bifidobacteria. Carbohydr. Polym. 2010, 82, 419–423. [Google Scholar] [CrossRef]
- Madhukumar, M.S.; Muralikrishna, G. Fermentation of xylo-oligosaccharides obtained from wheat bran and Bengal gram husk by lactic acid bacteria and bifidobacteria. J. Food Sci. Technol. 2012, 49, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Zeybek, N.; Rastall, R.A.; Buyukkileci, A.O. Utilization of xylan-type polysaccharides in co-culture fermentations of Bifidobacterium and Bacteroides species. Carbohydr. Polym. 2020, 236, 116076. [Google Scholar] [CrossRef]
- Sluiter, A.A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–9.





| Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) | Extractive (%) | References |
|---|---|---|---|---|---|
| 37.26 | 14.62 | 31.64 | 6.69 | 1.34 | [17] |
| 35.8 | 19.9 * | 32.1 | - | - | [18] |
| 59.7 | 22.1 | 18.1 | - | - | [19] |
| 47.6 | 28.1 | 13.1 | - | - | [20] |
| 28.3 | 36.6 | 35.1 | - | - | [21] |
| Inducer (Xylan:DP2:DP3) * | 1:0:0 | 1:0.15:0.04 | 1:0.28:0.04 | 1:1:0.04 | 1:1:0.14 | 1:1:0.26 |
|---|---|---|---|---|---|---|
| Protein concentration (mg/mL) | 1.03 ± 0.04 | 0.85 ± 0.02 | 0.88 ± 0.04 | 1.09 ± 0.25 | 1.27 ± 0.02 | 1.25 ± 0.08 |
| Activity (U/mL) | 50.8 ± 9.3 | 85.1 ± 4.5 | 89.1 ± 49.5 | 127.2 ± 54.4 | 135.6 ± 27.8 | 121.2 ± 15.8 |
| Specific activity (U/mg) | 49.6 ± 11.0 | 100.8 ± 7.5 | 102.1 ± 60.1 | 114.4 ± 41.1 | 106.1 ± 20.1 | 96.5 ± 11.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thirametoakkhara, C.; Hong, Y.-C.; Lerkkasemsan, N.; Shih, J.-M.; Chen, C.-Y.; Lee, W.-C. Application of Endoxylanases of Bacillus halodurans for Producing Xylooligosaccharides from Empty Fruit Bunch. Catalysts 2023, 13, 39. https://doi.org/10.3390/catal13010039
Thirametoakkhara C, Hong Y-C, Lerkkasemsan N, Shih J-M, Chen C-Y, Lee W-C. Application of Endoxylanases of Bacillus halodurans for Producing Xylooligosaccharides from Empty Fruit Bunch. Catalysts. 2023; 13(1):39. https://doi.org/10.3390/catal13010039
Chicago/Turabian StyleThirametoakkhara, Chanakan, Yi-Cheng Hong, Nuttapol Lerkkasemsan, Jian-Mao Shih, Chien-Yen Chen, and Wen-Chien Lee. 2023. "Application of Endoxylanases of Bacillus halodurans for Producing Xylooligosaccharides from Empty Fruit Bunch" Catalysts 13, no. 1: 39. https://doi.org/10.3390/catal13010039
APA StyleThirametoakkhara, C., Hong, Y. -C., Lerkkasemsan, N., Shih, J. -M., Chen, C. -Y., & Lee, W. -C. (2023). Application of Endoxylanases of Bacillus halodurans for Producing Xylooligosaccharides from Empty Fruit Bunch. Catalysts, 13(1), 39. https://doi.org/10.3390/catal13010039