Influences of Co-Content on the Physico-Chemical and Catalytic Properties of Perovskite GdCoxFe1−xO3 in CO Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
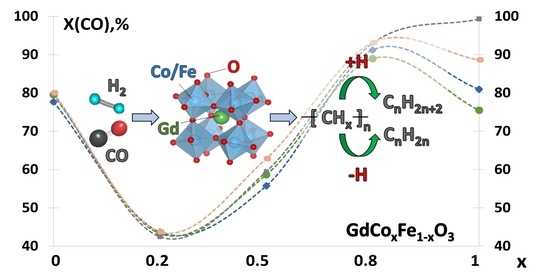
2.2. CO Hydrogenation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brussino, P.; Banús, E.D.; Ulla, M.A.; Bortolozzi, J.P. NiO-based ceramic structured catalysts for ethylene production: Substrates and active sites. Catal. Today 2022, 383, 84–92. [Google Scholar] [CrossRef]
- Coomb, D. EVP, Global Olefins & Polyolefins, Chemical Intensity Conference. 2016. Available online: https://www.lyondellbasell.com/globalassets/investors/events/2016/160314-lyb-gs-chemical-intensity-conference-final.pdf (accessed on 15 March 2016).
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K. Influence of reduction temperature on the catalytic behavior of Co/TiO2 catalysts for CH4/CO2 reforming and its relation with titania bulk crystal structure. J. Catal. 2005, 230, 75–85. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Zhu, Y.A.; Tong, G.; Zhang, J.; Engelbrekt, C.; Ulstrup, J.; Zhu, K.; Zhou, X. Tuning the composition of metastable CoxNiyMg100−x−y(OH)(OCH3) nanoplates for optimizing robust methane dry reforming catalyst. J. Catal. 2015, 330, 106–119. [Google Scholar] [CrossRef]
- Seiyama, T. Total Oxidation of Hydrocarbons on Perovskite Oxides. Catal. Rev. 1992, 34, 281–300. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W., Jr.; Remeika, J.P.; Gallagher, P.K. Perovskite oxides: Materials science in catalysis. Science 1977, 195, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.G.; Li, J.; Bennett, J.W.; Rappe, A.M.; Seshadri, R.; Scott, S.L. A Pd-doped perovskite catalyst, BaCe1−xPdxO3, for CO oxidation. J. Catal. 2007, 249, 349–358. [Google Scholar] [CrossRef]
- Bashan, V.; Ust, Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation. Int. J. Energy Res. 2019, 43, 7755–7789. [Google Scholar] [CrossRef]
- Saada, Y.; Álvarez-Serrano, I.; López, M.L.; Hidouria, M. Structural and dielectric characterization of new lead-free perovskites in the (SrTiO3)–(BiFeO3) system. Ceram. Int. 2016, 42, 8962–8973. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Leontiou, A.A.; Ladavos, A.K.; Pomonis, P.J. Characterization and catalytic investigation of NO + CO reaction on perovskites of the general formula LaxM1−xFeO3 (M = Sr and/or Ce) prepared via a reverse micelles microemulsion route. Appl. Catal. A Gen. 2006, 309, 254–262. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Jayaram, R.V. Liquid phase catalytic transfer hydrogenation of aromatic nitro compounds on perovskites prepared by microwave irradiation. Appl. Catal. A Gen. 2003, 252, 225–230. [Google Scholar] [CrossRef]
- Goldwasser, M.R.; Dorantes, V.E.; Pérez-Zurit, M.J.; Sojo, P.R.; Cubeiro, M.L.; Pietri, E.; González-Jiménez, F.; NgLee, Y.; Moronta, D. Modified iron perovskites as catalysts precursors for the conversion of syngas to low molecular weight alkenes. J. Mol. Catal. A Chem. 2003, 193, 227–236. [Google Scholar] [CrossRef]
- Silva-Santana, M.C.; daSilva, C.A.; Barrozo, P.; Plaza, E.J.R.; Valladares, L.d.; Moreno, N.O. Magnetocaloric and magnetic properties of SmFe0.5Mn0.5O3 complex perovskite. J. Magn. Magn. Mater. 2016, 401, 612–617. [Google Scholar] [CrossRef]
- Kesić, Ž.; Lukić, I.; Zdujić, M.; Jovalekić, Č.; Veljković, V.; Skala, D. Assessment of CaTiO3, CaMnO3, CaZrO3 and Ca2Fe2O5 perovskites as heterogeneous base catalysts for biodiesel synthesis. Fuel Process. Technol. 2016, 143, 162–168. [Google Scholar] [CrossRef]
- Forni, L.; Rossetti, I. Catalytic combustion of hydrocarbons over perovskites. Appl. Catal. B Environ. 2002, 38, 29–37. [Google Scholar] [CrossRef]
- Galasso, F.S. Structure of Perovskite-Type Compounds; A Volume in International Series of Monographs in Solid State Physics; Elsevier Inc.: Amsterdam, The Netherlands, 1969; pp. 3–49. [Google Scholar] [CrossRef]
- Moure, C.; Peña, O. Recent advances in perovskites: Processing and properties. Prog. Solid State Chem. 2015, 43, 123–148. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292. [Google Scholar] [CrossRef]
- Lopatin, S.I.; Zvereva, I.A.; Chislova, I.V. Vaporization and Thermodynamic Properties of GdFeO3 and GdCoO3 Complex Oxides. Russ. J. Gen. Chem. 2020, 90, 1495–1500. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Oh, J.H.; Kwon, B.W.; Cho, J.; Lee, C.H.; Kim, M.K.; Choi, S.H.; Yoon, S.P.; Han, J.; Nam, S.W.; Kim, J.Y.; et al. Importance of Exsolution in Transition-Metal (Co, Rh, and Ir)-Doped LaCrO3 Perovskite Catalysts for Boosting Dry Reforming of CH4 Using CO2 for Hydrogen Production. Ind. Eng. Chem. Res. 2019, 58, 6385–6393. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Zhao, T.; Yu, H.; Li, M. Dry reforming of methane over bimetallic Ni-Co catalyst prepared from La(CoxNi1−x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B Environ. 2019, 245, 302–313. [Google Scholar] [CrossRef]
- Gao, S.; Liu, N.; Liu, J.; Chen, W.; Liang, X.; Yuan, Y. Synthesis of higher alcohols by CO hydrogenation over catalysts derived from LaCo1−xMnxO3 perovskites: Effect of the partial substitution of Co by Mn. Fuel 2020, 261, 116415. [Google Scholar] [CrossRef]
- Zhu, Q.; Cheng, H.; Zou, X.; Lu, X.; Xu, Q.; Zhou, Z. Synthesis, characterization, and catalytic performance of La0.6Sr0.4NixCo1−xO3 perovskite catalysts in dry reforming of coke oven gas. Chin. J. Catal. 2015, 36, 915–924. [Google Scholar] [CrossRef]
- Bedel, L.; Roger, A.C.; Estournes, C.; Kiennemann, A. Co0 from partial reduction of La(Co,Fe)O3 perovskites for Fischer-Tropsch synthesis. Catal. Today. 2003, 85, 207–218. [Google Scholar] [CrossRef]
- Bedel, L.; Roger, A.C.; Rehspringer, J.L.; Zimmermann, Y.; Kiennemann, A. La(1-y)Co0.4Fe0.6O3-δ perovskite oxides as catalysts for Fischer-Tropsch synthesis. J. Catal. 2005, 235, 279–294. [Google Scholar] [CrossRef]
- Bedel, L.; Roger, A.C.; Rehspringer, J.L.; Kiennemann, A. Structure-controlled La-Co-Fe perovskite precursors for higher C2-C4 olefins selectivity in Fischer-Tropsch synthesis. Stud. Surf. Sci. Catal. 2004, 147, 319–324. [Google Scholar] [CrossRef]
- Sheshko, T.F.; Markova, E.B.; Sharaeva, A.A.; Kryuchkova, T.A.; Zvereva, I.A.; Chislova, I.V.; Yafarova, L.V. Carbon Monoxide Hydrogenation over Gd(Fe/Mn)O3 Perovskite-Type Catalysts. Pet. Chem. 2019, 59, 1307–1313. [Google Scholar] [CrossRef]
- Escalona, N.; Fuentealba, S.; Pecchi, G. Fischer-Tropsch synthesis over LaFe1−xCoxO3 perovskites from a simulated biosyngas feed. Appl. Catal. A Gen. 2010, 381, 253–260. [Google Scholar] [CrossRef]
- Ding, M.; Yang, Y.; Wu, B.; Li, Y.; Wang, T.; Ma, L. Study on reduction and carburization behaviors of iron phases for iron-based Fischer-Tropsch synthesis catalyst. Energy Proc. 2014, 61, 2267–2270. [Google Scholar] [CrossRef] [Green Version]
- Sheshko, T.F.; Kryuchkova, T.A.; Yafarova, L.V.; Borodina, E.M.; Serov, Y.M.; Zvereva, I.A.; Cherednichenko, A.G. Gd-Co-Fe perovskite mixed oxides as catalysts for dry reforming of methane. Sustain. Chem. Pharm. 2022, 30, 100897. [Google Scholar] [CrossRef]
- Yafarova, L.V.; Mamontov, G.V.; Chislova, I.V.; Silyukov, O.I.; Zvereva, I.A. The Effect of Transition Metal Substitution in the Perovskite-Type Oxides on the Physicochemical Properties and the Catalytic Performance in Diesel Soot Oxidation. Catalysts 2021, 11, 1256. [Google Scholar] [CrossRef]
- Sheshko, T.F.; Serov, Y.M.; Kryuchkova, T.A.; Khayrullina, I.A.; Chislova, I.V.; Yafarova, L.V.; Zvereva, I.A. Study of effect of preparation method and composition on the catalytic properties of complex oxides (Gd,Sr)n+1FenO3n+1 for dry reforming of methane. Nanotechnol. Russ. 2017, 12, 174–184. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Chu, B.; Yi, Y.; Qin, Z.; Fan, M.; Qin, Q.; He, H.; Zhang, L.; Dong, L.; et al. Catalytic reduction of NO by CO over B-site partially substituted LaM0.25Co0.75O3 (M = Cu, Mn, Fe) perovskite oxide catalysts: The correlation between physicochemical properties and catalytic performance. Appl. Catal. A Gen. 2018, 568, 43–53. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Eloy, P.; Cadús, L.E. La1−xCaxCoO3 perovskite-type oxides: Identification of the surface oxygen species by XPS. Appl. Surf. Sci. 2006, 253, 1489–1493. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Wang, Y.; Zhang, F.; Liu, X.; Guo, Y.; Lu, G. Nanocasted synthesis of mesoporous LaCoO3 perovskite with extremely high surface area and excellent activity in methane combustion. J. Phys. Chem. C 2008, 112, 15293–15298. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Z.; Li, Y.; Yu, X.; Zhao, L.; Li, J.; Wei, Y.; Liu, J. Synthesis and catalytic performance of macroporous La1−xCexCoO3 perovskite oxide catalysts with high oxygen mobility for catalytic combustion of soot. J. Rare Earths Chin. Soc. Rare Earths 2020, 38, 584–593. [Google Scholar] [CrossRef]
- Kryuchkova, T.A.; Kost’, V.V.; Sheshko, T.F.; Chislova, I.V.; Yafarova, L.V.; Zvereva, I.A. Effect of Cobalt in GdFeO3 Catalyst Systems on Their Activity in the Dry Reforming of Methane to Synthesis Gas. Pet. Chem. 2020, 60, 609–615. [Google Scholar] [CrossRef]
- Chislova, I.V.; Matveeva, A.A.; Volkova, A.V.; Zvereva, I.A. Sol-gel synthesis of nanostructured perovskite-like gadolinium ferrites. Glass Phys. Chem. 2011, 37, 653–660. [Google Scholar] [CrossRef]
- Yafarova, L.V.; Chislova, I.V.; Zvereva, I.A.; Kryuchkova, T.A.; Kost, V.V.; Sheshko, T.F. Sol–gel synthesis and investigation of catalysts on the basis of perovskite-type oxides GdMO3 (M = Fe, Co). J. Sol. Gel Sci. Technol. 2019, 92, 264–272. [Google Scholar] [CrossRef]
- Osman, M.E.; Maximov, V.V.; Dorokhov, V.S.; Mukhin, V.M.; Sheshko, T.F.; Kooyman, P.J.; Kogan, V.M. Carbon-Supported KCoMoS2 for Alcohol Synthesis from Synthesis Gas. Catalysts 2021, 11, 1321. [Google Scholar] [CrossRef]












| Compound | XRD Crystallite Size, nm | SBET (m2/g) | Content (at %) | Fe/Co | |||
|---|---|---|---|---|---|---|---|
| Gd | Fe | Co | O | ||||
| GdFeO3 | 53.4 | 9.9 | 18.53 | 19.15 | - | 62.32 | - |
| GdCo0.2Fe0.8O3 | 47.3 | 3.3 | 17.58 | 16.35 | 4.67 | 61.40 | 3.5 |
| GdCo0.5Fe0.5O3 | 50.5 | 2.7 | 17.43 | 8.77 | 9.49 | 64.31 | 0.92 |
| GdCo0.8Fe0.2O3 | 65.2 | 3.1 | 16.84 | 3.96 | 17.21 | 61.98 | 0.23 |
| GdCoO3 | 59.9 | 2.3 | 16.02 | - | 17.80 | 66.18 | - |
| Compound | Fe | Co | Ratio | ||
|---|---|---|---|---|---|
| Co2+/ ∑ Con+ | Fe2+/ Fe2+ + Fe3 | Os/Ol | |||
| GdFeO3 | +3 | - | - | 0.64 | 1.52 |
| GdCo0.2Fe0.8O3 | +2; +3 | +2; +3 | 0.51 | 0.43 | 0.59 |
| GdCo0.5Fe0.5O3 | +2; +3 | +2; +3 | 0.50 | 0.58 | 0.81 |
| GdCo0.8Fe0.2O3 | +2; +3 | +2; +3 | 0.13 | 0.58 | 0.82 |
| GdCoO3 | - | +2; +3 | 0.47 | - | 0.83 |
| Compound | GdFeO3 | GdCo0.2Fe0.8O3 | GdCo0.5Fe0.5O3 | GdCo0.8Fe0.2O3 | GdCoO3 |
|---|---|---|---|---|---|
| Co2+/∑ Con+ | - | 0.52 | 0.50 | 0.13 | 0.47 |
| Fe2+/Fe2+ + Fe3+ | 0.64 | 0.43 | 0.58 | 0.58 | - |
| Os/Ol | 1.52 | 0.59 | 0.81 | 0.82 | 0.83 |
| α(CO), % | 80 | 44 | 63 | 93 | 89 |
| S(CnH2n), % | 27.1 | 38.4 | 26.4 | 2.4 | 0.55 |
| Ea(CH4), kJ/mol | 70 | 77 | 99 | 177/252 | 159 |
| ln K0(CH4) | 5.7 | 5.6 | 11.3 | 29.6/46.6 | 25.1 |
| R2 | 0.96 | 0.97 | 0.99 | 0.99/0.99 | 0.99 |
| Ea(C2H4), kJ/mol | 112 | 88 | 115/186 | 112/244 | 99/226 |
| ln K0(C2H4) | 18.5 | 6.7 | 12.9/28.2 | 15.0/43.1 | 11.5/37.1 |
| R2 | 0.97 | 0.97 | 0.96/0.99 | 0.96/0.99 | 0.98/0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borodina, E.M.; Yafarova, L.V.; Kryuchkova, T.A.; Sheshko, T.F.; Cherednichenko, A.G.; Zvereva, I.A. Influences of Co-Content on the Physico-Chemical and Catalytic Properties of Perovskite GdCoxFe1−xO3 in CO Hydrogenation. Catalysts 2023, 13, 8. https://doi.org/10.3390/catal13010008
Borodina EM, Yafarova LV, Kryuchkova TA, Sheshko TF, Cherednichenko AG, Zvereva IA. Influences of Co-Content on the Physico-Chemical and Catalytic Properties of Perovskite GdCoxFe1−xO3 in CO Hydrogenation. Catalysts. 2023; 13(1):8. https://doi.org/10.3390/catal13010008
Chicago/Turabian StyleBorodina, Elizaveta M., Liliya V. Yafarova, Tatiana A. Kryuchkova, Tatiana F. Sheshko, Alexander G. Cherednichenko, and Irina A. Zvereva. 2023. "Influences of Co-Content on the Physico-Chemical and Catalytic Properties of Perovskite GdCoxFe1−xO3 in CO Hydrogenation" Catalysts 13, no. 1: 8. https://doi.org/10.3390/catal13010008
APA StyleBorodina, E. M., Yafarova, L. V., Kryuchkova, T. A., Sheshko, T. F., Cherednichenko, A. G., & Zvereva, I. A. (2023). Influences of Co-Content on the Physico-Chemical and Catalytic Properties of Perovskite GdCoxFe1−xO3 in CO Hydrogenation. Catalysts, 13(1), 8. https://doi.org/10.3390/catal13010008