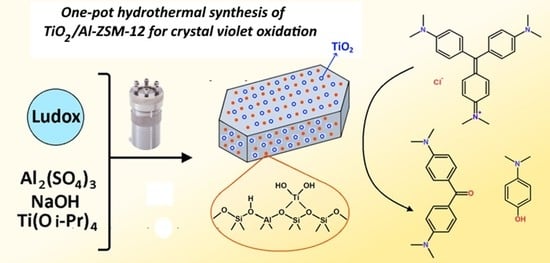
Synthesis and Investigation of Zeolite TiO2/Al-ZSM-12 Structure and Properties
Abstract
:1. Introduction
2. Results
2.1. X-ray Diffraction (XRD)
2.2. N2 Adsorption–Desorption
2.3. Scanning and Transmission Electron Microscopy
2.4. Fourier Transform Infrared Spectroscopy (FTIR), Raman Spectroscopy (Raman), UV–Visible Diffuse Reflectance Spectroscopy (DRS UV-Vis), Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS), and Photoluminescence Spectroscopy (PL Spectroscopy)
2.5. Solid-State Nuclear Magnetic Resonance Spectroscopy (SS NMR) on Different Nuclei
2.6. Thermogravimetric (TG), Differential Scanning Calorimetry (DSC), and Differential Thermal Analysis (DTA)
2.7. Temperature-Programmed Ammonia Desorption (NH3–TPD)
2.8. Fourier Transform Infrared Spectroscopy of Adsorbed Pyridine (FTIR-Py), 2,6-di-Tert-Butyl-Pyridine at External Surface (FTIR-2,6-dTBP), Deuterated Acetonitrile (FTIR-CD3CN), and Investigation of Acidity Method Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS-Acid)
2.9. Catalytic Tests
3. Materials and Methods
3.1. Synthesis of Zeolites Al-ZSM-12 and TiO2/Al-ZSM-12
- 20.83Na2O:1Al2O3:280SiO2: 35.3 template:3501.6H2O—for Al-ZSM-12
- 20.83Na2O:1Al2O3:1TiO2:280SiO2:35.3 template:3501.6H2O—for TiO2/Al-ZSM-12
3.2. Characterization
3.3. The Technique of the Catalytic Experiments
- Suspension preparation. 2 mL of water was added to a 1.5 mg weighed portion of the test sample, after which stirring was carried out for 30 min until a homogeneous suspension was formed;
- Dye adsorption on the surface of the photocatalyst. The aqueous solution of crystal violet dye (40 μL, 400 mg∙L−1) was added to the resulting suspension. The suspension was vigorously stirred in the darkness for 45 min to establish the adsorption–desorption equilibrium and prevent premature photocatalytic decomposition of the dye in the light. Then, hydrogen peroxide (40 μL, 3% solution) was added to the suspension, if necessary;
- Photocatalytic activity measurement. After the adsorption–desorption equilibrium was established, the zeolite suspension was irradiated with an Ocean Optics HPX-2000 xenon lamp (the radiation power of 1.52 mW, measured in the range of 200–1100 nm with an integrated optical power meter). The spectrophotometric analysis of the suspension was performed using an Ocean Optics QE65000 spectrophotometer. The dye concentration was calculated from the optical density at the absorption maximum (λ~590 nm) minus the background absorption (λ = 700 nm) of the spectrum of the catalyst suspension without the dye. The control experiment on the decomposition of the dye using hydrogen peroxide in the light was carried out in the absence of a catalyst.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramsay, J.D.F.; Kallus, S. Zeolite Membranes. Membr. Sci. Technol. 2000, 6, 373–395. [Google Scholar]
- Zeolite Catalysts Come into Focus. Nat. Mater. 2020, 19, 1037. [CrossRef] [PubMed]
- Naranov, E.R.; Dement’ev, K.I.; Gerzeliev, I.M.; Kolesnichenko, N.V.; Roldugina, E.A.; Maksimov, A.L. The Role of Zeolite Catalysis in Modern Petroleum Refining: Contribution from Domestic Technologies. Pet. Chem. 2019, 59, 247–261. [Google Scholar] [CrossRef]
- Naranov, E.R.; Golubev, O.V.; Guseva, A.I.; Nikulshin, P.A.; Maksimov, A.L.; Karakhanov, E.A. Hydrotreating of Middle-Distillate Fraction on Sulfide Catalysts Containing Crystalline Porous Aluminosilicates. Pet. Chem. Neft. 2017, 57, 965–5441. [Google Scholar] [CrossRef]
- Fairuzov, D.; Gerzeliev, I.; Maximov, A.; Naranov, E. Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology. Catalysts 2021, 11, 833. [Google Scholar] [CrossRef]
- Shavaleev, D.A.; Pavlov, M.L.; Basimova, R.A.; Sadovnikov, A.A.; Sudin, V.V.; Smirnova, E.M.; Demikhova, N.R.; Grigor’ev, Y.V.; Maksimov, A.L.; Naranov, E.R. Synthesis of Modified Catalyst for Liquid Phase Alkylation of Benzene with Ethylene. Pet. Chem. 2020, 60, 1073–1079. [Google Scholar] [CrossRef]
- Sadovnikov, A.A.; Arapova, O.V.; Russo, V.; Maximov, A.L.; Murzin, D.Y. Synergy of Acidity and Morphology of Micro-/Mesoporous Materials in the Solid-Acid Alkylation of Toluene with 1-Decene. Ind. Eng. Chem. Res. 2022, 61, 1994–2009. [Google Scholar] [CrossRef]
- Bok, T.O.; Andriako, E.P.; Ivanova, I.I. Effect of Binder Content on the Properties of Nanocrystalline Zeolite BEA-Based Catalysts for Benzene Alkylation with Propylene. Pet. Chem. 2021, 61, 901–907. [Google Scholar] [CrossRef]
- Saab, R.; Polychronopoulou, K.; Anjum, D.H.; Charisiou, N.D.; Goula, M.A.; Hinder, S.J.; Baker, M.A.; Schiffer, A. Effect of SiO2/Al2O3 Ratio in Ni/Zeolite-Y and Ni-W/Zeolite-Y Catalysts on Hydrocracking of Heptane. Mol. Catal. 2022, 528, 112484. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Huang, Y.; Xiong, F.; Luo, R. Defect-Designed ZSM-12 Zeolites for Alkylation of Phenol with Tert-butyl Alcohol. Mol. Catal. 2022, 519, 112144. [Google Scholar] [CrossRef]
- Tsai, S.-T.; Chao, P.-H.; Liu, S.-B.; Tsai, T.-C. Synergism of Acidic Zeolite and Pt/Zeolite in Aromatics Transalkylation. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1183–1186. [Google Scholar]
- Lu, X.; Guo, Y.; Zhang, Y.; Ma, R.; Fu, Y.; Zhu, W. Enhanced Catalytic Activity of Pt/H-ZSM-12 via Alkaline Post-Treatment for the Hydroisomerization of n-Hexane. Microporous Mesoporous Mater. 2020, 306, 110459. [Google Scholar] [CrossRef]
- Carvalho, K.T.G.; Urquieta-Gonzalez, E.A. Microporous–Mesoporous ZSM-12 Zeolites: Synthesis by Using a Soft Template and Textural, Acid and Catalytic Properties. Catal. Today 2015, 243, 92–102. [Google Scholar] [CrossRef]
- Sanhoob, M.A.; Muraza, O.; Yoshioka, M.; Qamaruddin, M.; Yokoi, T. Lanthanum, Cerium, and Boron Incorporated ZSM-12 Zeolites for Catalytic Cracking of n -Hexane. J. Anal. Appl. Pyrolysis 2018, 129, 231–240. [Google Scholar] [CrossRef]
- Tsaplin, D.E.; Makeeva, D.A.; Kulikov, L.A.; Maksimov, A.L.; Karakhanov, E.A. Synthesis of ZSM-12 Zeolites with New Templates Based on Salts of Ethanolamines. Russ. J. Appl. Chem. 2018, 91, 1957–1962. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y.; Gong, Z.; Wang, B.; Zhu, Z.; Jiang, J.; Xu, H.; Sun, H.; Han, L.; Wu, P.; et al. Bolaform Molecules Directing Intergrown Zeolites. J. Phys. Chem. C 2018, 122, 9117–9126. [Google Scholar] [CrossRef]
- Mal, N.K.; Bhaumik, A.; Kumar, R.; Ramaswamy, A.V. Sn-ZSM-12, a New, Large Pore MTW Type Tin-Silicate Molecular Sieve: Synthesis, Characterization and Catalytic Properties in Oxidation Reactions. Catal. Lett. 1995, 33, 387–394. [Google Scholar] [CrossRef]
- Zhi, Y.-X.; Tuel, A.; Taarit, Y.B.; Naccache, C. Synthesis of Gallosilicates—MTW- Type Structure Zeolites: Evidence of Ga-Substituted T Atoms. Zeolites 1992, 12, 138–141. [Google Scholar] [CrossRef]
- Reddy, K.M.; Moudrakovski, I.; Sayari, A. VS-12: A Novel Large-Pore Vanadium Silicate with ZSM-12 Structure. J. Chem. Soc. Chem. Commun. 1994, 1491–1492. [Google Scholar] [CrossRef]
- Tuel, A. Synthesis, Characterization, and Catalytic Properties of the New TiZSM-12 Zeolite. Zeolites 1995, 15, 236–242. [Google Scholar] [CrossRef]
- Davis, R.J.; Liu, Z. Titania−Silica: A Model Binary Oxide Catalyst System. Chem. Mater. 1997, 9, 2311–2324. [Google Scholar] [CrossRef]
- Noguera, C. Chapter 2 Clean Oxide Surfaces: A Theoretical Review. Chem. Phys. Solid Surf. 2001, 9, 35–93. [Google Scholar]
- Ullah, R.; Liu, C.; Panezai, H.; Gul, A.; Sun, J.; Wu, X. Controlled Crystal Phase and Particle Size of Loaded-TiO2 Using Clinoptilolite as Support via Hydrothermal Method for Degradation of Crystal Violet Dye in Aqueous Solution. Arab. J. Chem. 2020, 13, 4092–4101. [Google Scholar] [CrossRef]
- Djellabi, R.; Giannantonio, R.; Falletta, E.; Bianchi, C.L. SWOT Analysis of Photocatalytic Materials towards Large Scale Environmental Remediation. Curr. Opin. Chem. Eng. 2021, 33, 100696. [Google Scholar] [CrossRef]
- Mesbah, M.; Sarraf, M.; Dabbagh, A.; Nasiri-Tabrizi, B.; Paria, S.; Banihashemian, S.M.; Bushroa, A.R.; Faraji, G.; Tsuzuki, T.; Madaah Hosseini, H.R. Synergistic Enhancement of Photocatalytic Antibacterial Effects in High-Strength Aluminum/TiO2 Nanoarchitectures. Ceram. Int. 2020, 46, 24267–24280. [Google Scholar] [CrossRef]
- Prado, R.; Erdocia, X.; Labidi, J. Effect of the Photocatalytic Activity of TiO2 on Lignin Depolymerization. Chemosphere 2013, 91, 1355–1361. [Google Scholar] [CrossRef]
- Aboagye, D.; Medina, F.; Contreras, S. Toward a Facile Depolymerization of Alkaline Lignin into High-Value Platform Chemicals via the Synergetic Combination of Mechanocatalysis with Photocatalysis or Fenton Process. Catal. Today, 2022; in press. [Google Scholar] [CrossRef]
- Machut, C.; Kania, N.; Léger, B.; Wyrwalski, F.; Noël, S.; Addad, A.; Monflier, E.; Ponchel, A. Fast Microwave Synthesis of Gold-Doped TiO2 Assisted by Modified Cyclodextrins for Photocatalytic Degradation of Dye and Hydrogen Production. Catalysts 2020, 10, 801. [Google Scholar] [CrossRef]
- Torres, J.C.; Cardoso, D. The Influence of Gel Alkalinity in the Synthesis and Physicochemical Properties of the Zeolite [Ti,Al]-Beta. Microporous Mesoporous Mater. 2008, 113, 204–211. [Google Scholar] [CrossRef]
- Subagyo, R.; Tehubijuluw, H.; Utomo, W.P.; Rizqi, H.D.; Kusumawati, Y.; Bahruji, H.; Prasetyoko, D. Converting Red Mud Wastes into Mesoporous ZSM-5 Decorated with TiO2 as an Eco-Friendly and Efficient Adsorbent-Photocatalyst for Dyes Removal. Arab. J. Chem. 2022, 15, 103754. [Google Scholar] [CrossRef]
- Huang, F.; Hao, H.; Sheng, W.; Dong, X.; Lang, X. Cooperative Photocatalysis of Dye–Ti-MCM-41 with Trimethylamine for Selective Aerobic Oxidation of Sulfides Illuminated by Blue Light. J. Colloid Interface Sci. 2023, 630, 921–930. [Google Scholar] [CrossRef]
- Kulikov, L.A.; Tsaplin, D.E.; Knyazeva, M.I.; Levin, I.S.; Kardashev, S.V.; Filippova, T.Y.; Maksimov, A.L.; Karakhanov, E.A. Effect of Template Structure on the Zeolite ZSM-12 Crystallization Process Characteristics. Pet. Chem. 2019, 59, S60–S65. [Google Scholar] [CrossRef]
- Kamimura, Y.; Iyoki, K.; Elangovan, S.P.; Itabashi, K.; Shimojima, A.; Okubo, T. OSDA-Free Synthesis of MTW-Type Zeolite from Sodium Aluminosilicate Gels with Zeolite Beta Seeds. Microporous Mesoporous Mater. 2012, 163, 282–290. [Google Scholar] [CrossRef]
- Akyalcin, S.; Akyalcin, L.; Bjørgen, M. Optimization of Desilication Parameters of Low-Silica ZSM-12 by Taguchi Method. Microporous Mesoporous Mater. 2019, 273, 256–264. [Google Scholar] [CrossRef]
- Ogura, M.; Nakata, S.; Kikuchi, E.; Matsukata, M. Effect of NH+4 Exchange on Hydrophobicity and Catalytic Properties of Al-Free Ti–Si–Beta Zeolite. J. Catal. 2001, 199, 41–47. [Google Scholar] [CrossRef]
- Gomez, S.; Marchena, C.L.; Pizzio, L.; Pierella, L. Preparation and Characterization of TiO2/HZSM-11 Zeolite for Photodegradation of Dichlorvos in Aqueous Solution. J. Hazard. Mater. 2013, 258, 19–26. [Google Scholar] [CrossRef]
- Reddy, J.S.; Kumar, R.; Csicsery, S.M. Synthesis, Characterization, and Catalytic Properties of Metallo-Titanium Silicate Molecular Sieves with MEL Topology. J. Catal. 1994, 145, 73–78. [Google Scholar] [CrossRef]
- Zhu, H.-B.; Xia, Q.-H.; Guo, X.-T.; Su, K.-X.; Hu, D.; Ma, X.; Zeng, D.; Deng, F. Synthesis and Structure-Directing Effect of Piperazinium Hydroxides Derived from Piperazines for the Formation of Porous Zeolites. Mater. Lett. 2006, 60, 2161–2166. [Google Scholar] [CrossRef]
- Wu, W.; Wu, W.; Kikhtyanin, O.V.; Li, L.; Toktarev, A.V.; Ayupov, A.B.; Khabibulin, J.F.; Echevsky, G.V.; Huang, J. Methylation of Naphthalene on MTW-Type Zeolites. Influence of Template Origin and Substitution of Al by Ga. Appl. Catal. A Gen. 2010, 375, 279–288. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Zhu, L.; Chen, S.; Zhang, Y.; Tang, Y.; Zhu, Y.; Li, Y. Synthesis of Nano Titania Particles Embedded in Mesoporous SBA-15: Characterization and Photocatalytic Activity. J. Hazard. Mater. 2006, 137, 952–958. [Google Scholar] [CrossRef]
- Domoroshchina, E.; Kravchenko, G.; Kuz’micheva, G.; Markova, E.; Zhukova, A.; Pirutko, L.; Khramov, E.; Dorokhov, A.; Koroleva, A. The Role of the Compositions of HZSM-5 Zeolites Modified with Nanosized Anatase in Propane and Ethanol Conversion. Catal. Today 2022, 397, 511–525. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, K.; Feng, Z.; Li, G.; Guo, M.; Fan, F.; Li, C. A Thorough Investigation of the Active Titanium Species in TS-1 Zeolite by In Situ UV Resonance Raman Spectroscopy. Chem.—Eur. J. 2012, 18, 13854–13860. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Cao, Y.; Guo, Z.; Jia, Q.; Tian, F.; Liu, L. The Roles of Different Titanium Species in TS-1 Zeolite in Propylene Epoxidation Studied by in Situ UV Raman Spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 190–196. [Google Scholar] [CrossRef]
- Halasz, I.; Agarwal, M.; Senderov, E.; Marcus, B. Efficient Oxyfunctionalization of N-Hexane by Aqueous H2O2 over a New TS-PQTM Catalyst. Catal. Today 2003, 81, 227–245. [Google Scholar] [CrossRef]
- Yamamoto, K.; Borjas García, S.E.; Muramatsu, A. Zeolite Synthesis Using Mechanochemical Reaction. Microporous Mesoporous Mater. 2007, 101, 90–96. [Google Scholar] [CrossRef]
- Horikawa, H.; Iida, T.; Osuga, R.; Ohara, K.; Kondo, J.N.; Wakihara, T. Crystallization of Ti-Rich *BEA Zeolites by the Combined Strategy of Using Ti–Si Mixed Oxide Composites and Intentional Aluminum Addition/Post-Synthesis Dealumination. Cryst. Growth Des. 2018, 18, 2180–2188. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Tao, Y.F.; Lin, Z.; Liu, L.; Fan, X.X.; Wang, Y. Hydrothermal Synthesis and Morphological Evolution of Mesoporous Titania−Silica. J. Phys. Chem. C 2009, 113, 20335–20348. [Google Scholar] [CrossRef]
- Trong On, D.; Nguyen, S.; Hulea, V.; Dumitriu, E.; Kaliaguine, S. Mono- and Bifunctional MFI, BEA and MCM-41 Titanium-Molecular Sieves. Part 1. Synthesis and Characterization. Microporous Mesoporous Mater. 2003, 57, 169–180. [Google Scholar] [CrossRef]
- Carati, A.; Flego, C.; Previde Massara, E.; Millini, R.; Carluccio, L.; Parker, W.; Bellussi, G. Stability of Ti in MFI and Beta Structures: A Comparative Study. Microporous Mesoporous Mater. 1999, 30, 137–144. [Google Scholar] [CrossRef]
- Mazaj, M.; Zabukovec Logar, N.; Mali, G.; Novak Tušar, N.; Arčon, I.; Ristić, A.; Rečnik, A.; Kaučič, V. Synthesis and Structural Properties of Titanium Containing Microporous/Mesoporous Silicate Composite (Ti, Al)-Beta/MCM-48. Microporous Mesoporous Mater. 2007, 99, 3–13. [Google Scholar] [CrossRef]
- Ovejero, G.; Van Grieken, R.; Uguina, M.A.; Serrano, D.P.; Melero, J.A. Bifunctional Properties of Al-TS-1 Synthesized by Wetness Impregnation of Amorphous Al2O3-TiO2-SiO2 Solids Prepared by the Sol-Gel Method. Catal. Lett. 1996, 41, 69–78. [Google Scholar] [CrossRef]
- Ganapathy, S.; Gore, K.; Kumar, R.; Amoureux, J.-P. Multinuclear (27Al, 29Si, 47,49Ti) Solid-State NMR of Titanium Substituted Zeolite USY. Solid State Nucl. Magn. Reson. 2003, 24, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kirkpatrick, R.J. High-Temperature Multi-Nuclear NMR Investigation of Analcime. Am. Mineral. 1998, 83, 339–347. [Google Scholar] [CrossRef]
- García-Benjume, M.L.; Espitia-Cabrera, M.I.; Contreras-García, M.E. Hierarchical Macro-Mesoporous Structures in the System TiO2–Al2O3, Obtained by Hydrothermal Synthesis Using Tween-20® as a Directing Agent. Mater. Charact. 2009, 60, 1482–1488. [Google Scholar] [CrossRef]
- Nakabayashi, H. Properties of Acid Sites on TiO2–SiO2 and TiO2–Al2 O3 Mixed Oxides Measured by Infrared Spectroscopy. Bull. Chem. Soc. Jpn. 1992, 65, 914–916. [Google Scholar] [CrossRef]
- Kurmach, M.M.; Yaremov, P.S.; Skoryk, M.O.; Shvets, O.V. Effect of Introduction of B3+ or Al3+ Ions in the Structure of Ti-, Sn-, AND Zr-Containing Heirarchical Zeolites on the Concentration of Lewis and Brønsted Acid Centers. Theor. Exp. Chem. 2016, 52, 190–196. [Google Scholar] [CrossRef]
- Naranov, E.R.; Sadovnikov, A.A.; Bugaev, A.L.; Shavaleev, D.A.; Maximov, A.L. A Stepwise Fabrication of MFI Nanosheets in Accelerated Mode. Catal. Today 2021, 378, 149–157. [Google Scholar] [CrossRef]
- Panahian, Y.; Arsalani, N.; Nasiri, R. Enhanced Photo and Sono-Photo Degradation of Crystal Violet Dye in Aqueous Solution by 3D Flower like F-TiO2(B)/Fullerene under Visible Light. J. Photochem. Photobiol. A Chem. 2018, 365, 45–51. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalysts for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Li, X.; Bian, Z.; Fuhr, A.; Zhang, D.; Zhu, J. Highly Photocatalytic Activity of Brookite/Rutile TiO2 Nanocrystals with Semi-Embedded Structure. Appl. Catal. B Environ. 2016, 180, 551–558. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Xu, G.; Feng, Q.; Lv, J.; Qin, Y.; Zhang, Y.; Zheng, Z.; Wu, Y. Crystalline Orientation Preference for TiO2 Nanotube Arrays with Efficient Photoelectrochemical Properties. Phys. Lett. A 2018, 382, 2759–2762. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. Increased Photocatalytic Activity of NiO and ZnO in Photodegradation of a Model Drug Aqueous Solution: Effect of Coupling, Supporting, Particles Size and Calcination Temperature. J. Hazard. Mater. 2017, 321, 629–638. [Google Scholar] [CrossRef] [PubMed]











| Zeolite | SBET, m2·g−1 | VMICRO, cm3·g−1 | VTOTAL, cm3·g−1 | a Elemental Composition, mol/mol | b Degree of Crystallinity, % | |||
|---|---|---|---|---|---|---|---|---|
| Si/Al | Si/Ti | Si/Na | Ti/Al | |||||
| TiO2/Al-ZSM-12 | 266 | 0.098 | 0.14 | 80 | 384 | 160 | 0.2 | 83 |
| Al-ZSM-12 | 274 | 0.1 | 0.13 | 105 | - | 837 | - | 93 |
| Zeolite | The Number of Acid Sites | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRIFTS-Acid, % | |||||||||||||||
| LASs | BASs | Total | B/L | BASs | Total | BASs | Weak Sites (100–300 °C) | Strong Sites (300–500 °C) | Total | ||||||
| Weak | Medium | Strong | a LT, °C | Amount | b HT, °C | Amount | |||||||||
| TiO2/Al-ZSM-12 | 38 | 15 | 53 | 0.4 | 83.1 | 3.2 | 13.7 | 100 | 2.5 | 188 | 205 | 393 | 32 | 237 | 6.41 |
| Al-ZSM-12 | 5 | 40 | 45 | 8 | 30.8 | 8.8 | 60.4 | 100 | 3.1 | 183 | 80 | 363 | 117 | 197 | 0.68 |
| Adsorption Centers of Probe Molecules | Wave Number, cm−1 | |||
|---|---|---|---|---|
| FTIR-Py | FTIR-2,6-dTBP | FTIR-CD3CN | DRIFTS-Acid | |
| isolated Si–OH groups located on the outer surface of the zeolite | 3745 | 3743 | N.D. | N.D. |
| internal Si–OH | 3740–3710 | N.D. | N.D. | |
| extra framework Al–OH groups | 3665 (Al-ZSM-12) | N.D. | N.D. | |
| Si–O(H)–Al, Brønsted acid sites | 3615 and 1547 | 3608 and 1620 | 2277 (Al-ZSM-12) | 3723 (BW) |
| 3674 (BM) | ||||
| 3596 (BS) | ||||
| silanol nests, (Si-OH)n | 3520 | N.D. | 2266 (TiO2/Al-ZSM-12) | N.D. |
| Lewis acid sites, [AlO4]− | 1455 | N.D. | 2330 (Al-ZSM-12); | N.D. |
| 2325 (TiO2/Al-ZSM-12) | ||||
| TiO2/Al-ZSM-12 | H2O2 | TiO2/Al-ZSM-12 + H2O2 | |
|---|---|---|---|
| a PCA, %·min−1 | 0.041 | 0.112 | 0.157 |
| Nucleus | Rotation Angle, ° | Pulse Duration, µs | Number of Scans | Interval between Scans, s | External Standard (0 ppm) |
|---|---|---|---|---|---|
| 1H | 90 | 2.9 | 16 | 3 | NH4OH, 25% aqueous solution |
| 27Al | 15 | 0.8 | 1024 | 0.5 | Al(NO3)3, 1M aqueous solution |
| 29Si | 90 | 4 | 256 | 60 | Si(CH3)4 |
| 23Na | 30 | 2 | 1 | 2048 | NaCl, 1M aqueous solution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsaplin, D.E.; Ostroumova, V.A.; Kulikov, L.A.; Zolotukhina, A.V.; Sadovnikov, A.A.; Kryuchkov, M.D.; Egazaryants, S.V.; Maksimov, A.L.; Wang, K.; Luo, Z.; et al. Synthesis and Investigation of Zeolite TiO2/Al-ZSM-12 Structure and Properties. Catalysts 2023, 13, 216. https://doi.org/10.3390/catal13020216
Tsaplin DE, Ostroumova VA, Kulikov LA, Zolotukhina AV, Sadovnikov AA, Kryuchkov MD, Egazaryants SV, Maksimov AL, Wang K, Luo Z, et al. Synthesis and Investigation of Zeolite TiO2/Al-ZSM-12 Structure and Properties. Catalysts. 2023; 13(2):216. https://doi.org/10.3390/catal13020216
Chicago/Turabian StyleTsaplin, Dmitry E., Vera A. Ostroumova, Leonid A. Kulikov, Anna V. Zolotukhina, Alexey A. Sadovnikov, Michail D. Kryuchkov, Sergey V. Egazaryants, Anton L. Maksimov, Kaige Wang, Zhongyang Luo, and et al. 2023. "Synthesis and Investigation of Zeolite TiO2/Al-ZSM-12 Structure and Properties" Catalysts 13, no. 2: 216. https://doi.org/10.3390/catal13020216
APA StyleTsaplin, D. E., Ostroumova, V. A., Kulikov, L. A., Zolotukhina, A. V., Sadovnikov, A. A., Kryuchkov, M. D., Egazaryants, S. V., Maksimov, A. L., Wang, K., Luo, Z., & Naranov, E. R. (2023). Synthesis and Investigation of Zeolite TiO2/Al-ZSM-12 Structure and Properties. Catalysts, 13(2), 216. https://doi.org/10.3390/catal13020216