Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis
Abstract
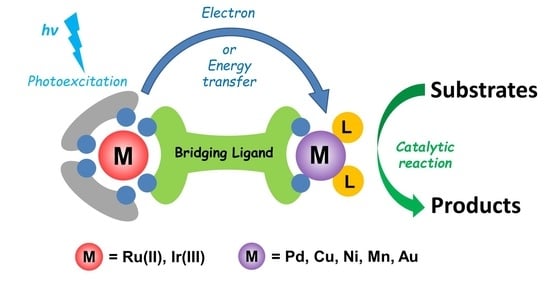
:1. Introduction
2. Heterobinuclear Photocatalysts
2.1. Heteropolynuclear Complexes for Hydrogen Photogeneration
2.2. Heterobinuclear Complexes for Photocaytalytic CO2 Reduction
2.3. Heterobinuclear Photocatalysts in Organic Synthesis
2.3.1. Dimerization of α-Methylstyrene
2.3.2. Other Pd-Catalyzed Reactions
2.3.3. Oxidation Reactions
2.3.4. Catalysis by Ni, Mn and Au Containing Complexes
3. Conclusions and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Yoon, T.P. Visible light photocatalysis: The development of photocatalytic radical ion cycloadditions. ACS Catal. 2013, 3, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon–Carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Ischay, M.A.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient visible light photocatalysis of [2+2] enone cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Lang, X.; Zhao, J.; Chen, X. Cooperative photoredox catalysis. Chem. Soc. Rev. 2016, 45, 3026–3038. [Google Scholar] [CrossRef]
- Chan, A.Y.; Perry, I.B.; Bissonnette, N.B.; Buksh, B.F.; Edwards, G.A.; Frye, L.I.; Garry, O.L.; Lavagnino, M.N.; Li, B.X.; Liang, Y.; et al. Metallaphotoredox: The merger of photoredox and transition metal catalysis. Chem. Rev. 2022, 122, 1485–1542. [Google Scholar] [CrossRef]
- Lipp, A.; Badir, S.O.; Molander, G.A. Stereoinduction in metallaphotoredox catalysis. Angew. Chem. Int. Ed. 2021, 60, 1714–1726. [Google Scholar] [CrossRef]
- Zhu, C.; Yue, H.; Jia, J.; Rueping, M. Nickel-catalyzed C-Heteroatom cross-coupling reactions under mild conditions via facilitated reductive elimination. Angew. Chem. Int. Ed. 2021, 60, 17810–17831. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.P.S.; Sarkar, S.; Gevorgyan, V. Visible light-induced transition metal catalysis. Chem. Rev. 2022, 122, 1543–1625. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Stephenson, C.R.J. Shining light on photoredox catalysis: Theory and synthetic applications. J. Org. Chem. 2012, 77, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Hammond, C. Catalytic formation of C(sp3)–F bonds via heterogeneous photocatalysis. ACS Catal. 2018, 8, 10321–10330. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef]
- Chen, J.; Cen, J.; Xu, X.; Li, X. The application of heterogeneous visible light photocatalysts in organic synthesis. Catal. Sci. Technol. 2016, 6, 349–362. [Google Scholar] [CrossRef]
- Pieber, B.; Shalom, M.; Antonietti, M.; Seeberger, P.H.; Gilmore, K. Continuous heterogeneous photocatalysis in serial micro-batch reactors. Angew. Chem. Int. Ed. 2018, 57, 9976–9979. [Google Scholar] [CrossRef]
- Dong, K.; Pezzetta, C.; Chen, Q.-C.; Kaushansky, A.; Agosti, A.; Bergamini, G.; Davidson, R.; Amirav, L. Nanorod photocatalysts for C−O cross-coupling reactions. ChemCatChem 2022, 14, e202200477. [Google Scholar] [CrossRef]
- Easun, T.L.; Alsindi, W.Z.; Towrie, M.; Ronayne, K.L.; Sun, X.-Z.; Ward, M.D.; George, M.W. Photoinduced energy transfer in a conformationally flexible Re(I)/Ru(II) dyad probed by time-resolved infrared spectroscopy: Effects of Conformation and Spatial Localization of Excited States. Inorg. Chem. 2008, 47, 5071–5078. [Google Scholar] [CrossRef]
- Schallenberg, D.; Neubauer, A.; Erdmann, E.; Tänzler, M.; Villinger, A.; Lochbrunner, S.; Seidel, W.W. Dinuclear Ru/Ni, Ir/Ni, and Ir/Pt complexes with bridging phenanthroline-5,6-dithiolate: Synthesis, structure, and electrochemical and photophysical Behavior. Inorg. Chem. 2014, 53, 8859–8873. [Google Scholar] [CrossRef] [PubMed]
- Troian-Gautier, L.; Moucheron, C. RutheniumII complexes bearing fused polycyclic ligands: From fundamental aspects to potential applications. Molecules 2014, 19, 5028–5087. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Kim, S.-Y.; Choi, C.M.; Kim, N.J.; Kim, C.H.; Cho, D.W.; Son, H.-J.; Pac, C.; Kang, S.O. Photophysics and excited-state properties of cyclometalated iridium(III)–platinum(II) and iridium(III)–iridium(III) bimetallic complexes bridged by dipyridylpyrazine. Inorg. Chem. 2017, 56, 5305–5315. [Google Scholar] [CrossRef] [PubMed]
- Laramée-Milette, B.; Hanan, G.S. Going against the flow: Os(II)-to-Ru(II) energy transfer in rod-like polypyridyl chromophore. Chem. Commun. 2017, 53, 10496–10499. [Google Scholar] [CrossRef] [PubMed]
- Zigler, D.F.; Morseth, Z.A.; White, T.A.; Canterbury, T.R.; Sayre, H.J.; Rodríguez-Corrales, J.Á.; Brennaman, M.K.; Brewer, K.J.; Papanikolas, J.M. Ultrafast kinetics of supramolecules with a Ru(II)- or Os(II)-polypyridyl light absorber, cis-Rh(III)Cl2-polypyridyl electron collector, and 2,3-bis(2-pyridyl)pyrazine bridge. Inorg. Chim. Acta 2017, 454, 266–274. [Google Scholar] [CrossRef]
- Erdmann, E.; Lütgens, M.; Lochbrunner, S.; Seidel, W.W. Ultrafast energy transfer in dinuclear complexes with bridging 1,10-phenanthroline-5,6-dithiolate. Inorg. Chem. 2018, 57, 4849–4863. [Google Scholar] [CrossRef]
- Staniszewska, M.; Kupfer, S.; Guthmuller, J. Effect of the catalytic center on the electron transfer dynamics in hydrogen-evolving ruthenium-based photocatalysts investigated by theoretical calculations. J. Phys. Chem. C 2019, 123, 16003–16013. [Google Scholar] [CrossRef]
- Zedler, L.; Müller, C.; Wintergerst, P.; Mengele, A.K.; Rau, S.; Dietzek-Ivanšić, B. Influence of the linker chemistry on the photoinduced charge-transfer dynamics of hetero-dinuclear photocatalysts. Chem. Eur. J. 2022, 28, e202200490. [Google Scholar] [CrossRef]
- Tamaki, Y.; Ishitani, O. Supramolecular photocatalysts for the reduction of CO2. ACS Catalysis 2017, 7, 3394–3409. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Ishitani, O.; Ishida, H. Reaction mechanisms of catalytic photochemical CO2 reduction using Re(I) and Ru(II) complexes. Coord. Chem. Rev. 2018, 373, 333–356. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Dar, A.H.; Shaikh, M.N.; Qurashi, A. Reticular-chemistry-inspired supramolecule design as a tool to achieve efficient photocatalysts for CO2 reduction. ACS Omega 2021, 6, 29291–29324. [Google Scholar] [CrossRef]
- Halpin, Y.; Pryce, M.T.; Rau, S.; Dini, D.; Vos, J.G. Recent progress in the development of bimetallic photocatalysts for hydrogen generation. Dalton Trans. 2013, 42, 16243–16254. [Google Scholar] [CrossRef] [PubMed]
- Manbeck, G.F.; Brewer, K.J. Photoinitiated electron collection in polyazine chromophores coupled to water reduction catalysts for solar H2 production. Coord. Chem. Rev. 2013, 257, 1660–1675. [Google Scholar] [CrossRef]
- Dini, D.; Pryce, M.T.; Schulz, M.; Vos, J.G. CHAPTER 12 Metallosupramolecular assemblies for application as photocatalysts for the production of solar fuels. In Functional Metallosupramolecular Materials; The Royal Society of Chemistry: London, UK, 2015; pp. 345–396. [Google Scholar]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; von Zelewsky, A. Ru(II) polypyridine complexes: Photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar] [CrossRef]
- Lutz, F.; Lorenzo-Parodi, N.; Schmidt, T.C.; Niemeyer, J. Heteroternary cucurbit [8] uril complexes as supramolecular scaffolds for self-assembled bifunctional photoredoxcatalysts. Chem. Commun. 2021, 57, 2887–2890. [Google Scholar] [CrossRef]
- Frischmann, P.D.; Mahata, K.; Würthner, F. Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem. Soc. Rev. 2013, 42, 1847–1870. [Google Scholar] [CrossRef]
- Bindra, G.S.; Schulz, M.; Paul, A.; Soman, S.; Groarke, R.; Inglis, J.; Pryce, M.T.; Browne, W.R.; Rau, S.; Maclean, B.J.; et al. The effect of peripheral bipyridine ligands on the photocatalytic hydrogen production activity of Ru/Pd catalysts. Dalton Trans. 2011, 40, 10812–10814. [Google Scholar] [CrossRef]
- Bindra, G.S.; Schulz, M.; Paul, A.; Groarke, R.; Soman, S.; Inglis, J.L.; Browne, W.R.; Pfeffer, M.G.; Rau, S.; MacLean, B.J.; et al. The role of bridging ligand in hydrogen generation by photocatalytic Ru/Pd assemblies. Dalton Trans. 2012, 41, 13050–13059. [Google Scholar] [CrossRef]
- Kowacs, T.; O’Reilly, L.; Pan, Q.; Huijser, A.; Lang, P.; Rau, S.; Browne, W.R.; Pryce, M.T.; Vos, J.G. Subtle changes to peripheral ligands enable high turnover numbers for photocatalytic hydrogen generation with supramolecular photocatalysts. Inorg. Chem. 2016, 55, 2685–2690. [Google Scholar] [CrossRef]
- Pan, Q.; Mecozzi, F.; Korterik, J.P.; Vos, J.G.; Browne, W.R.; Huijser, A. The critical role played by the catalytic moiety in the early-time photodynamics of hydrogen-generating bimetallic photocatalysts. ChemPhysChem 2016, 17, 2654–2659. [Google Scholar] [CrossRef]
- Kowacs, T.; Pan, Q.; Lang, P.; O’Reilly, L.; Rau, S.; Browne, W.R.; Pryce, M.T.; Huijser, A.; Vos, J.G. Supramolecular bimetallic assemblies for photocatalytic hydrogen generation from water. Faraday Discuss. 2015, 185, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Manbeck, G.F.; Wimer, D.G.; Brewer, K.J. A new RuIIRhIII bimetallic with a single Rh–Cl bond as a supramolecular photocatalyst for proton reduction. Chem. Commun. 2015, 51, 12966–12969. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.M.; Arachchige, S.M.; Brewer, K.J. Enhancement of solar fuel production schemes by using a Ru,Rh,Ru supramolecular photocatalyst containing hydroxide labile ligands. Chem. Eur. J. 2015, 21, 16948–16954. [Google Scholar] [CrossRef] [PubMed]
- Canterbury, T.R.; Arachchige, S.M.; Brewer, K.J.; Moore, R.B. A new hydrophilic supramolecular photocatalyst for the production of H2 in aerobic aqueous solutions. Chem. Commun. 2016, 52, 8663–8666. [Google Scholar] [CrossRef]
- White, J.K.; Brewer, K.J. A new Ru,Ru,Pt supramolecular architecture for photocatalytic H2 production. Chem. Commun. 2015, 51, 16123–16126. [Google Scholar] [CrossRef] [PubMed]
- Karnahl, M.; Kuhnt, C.; Ma, F.; Yartsev, A.; Schmitt, M.; Dietzek, B.; Rau, S.; Popp, J. Tuning of photocatalytic hydrogen production and photoinduced intramolecular electron transfer rates by regioselective bridging ligand substitution. ChemPhysChem 2011, 12, 2101–2109. [Google Scholar] [CrossRef]
- Pfeffer, M.G.; Kowacs, T.; Wächtler, M.; Guthmuller, J.; Dietzek, B.; Vos, J.G.; Rau, S. Optimization of hydrogen-evolving photochemical molecular devices. Angew. Chem. Int. Ed. 2015, 54, 6627–6631. [Google Scholar] [CrossRef]
- Pfeffer, M.G.; Schäfer, B.; Smolentsev, G.; Uhlig, J.; Nazarenko, E.; Guthmuller, J.; Kuhnt, C.; Wächtler, M.; Dietzek, B.; Sundström, V.; et al. Palladium versus platinum: The metal in the catalytic center of a molecular photocatalyst determines the mechanism of the hydrogen production with visible light. Angew. Chem. Int. Ed. 2015, 54, 5044–5048. [Google Scholar] [CrossRef]
- Mengele, A.K.; Kaufhold, S.; Streb, C.; Rau, S. Generation of a stable supramolecular hydrogen evolving photocatalyst by alteration of the catalytic center. Dalton Trans. 2016, 45, 6612–6618. [Google Scholar] [CrossRef]
- Pfeffer, M.G.; Zedler, L.; Kupfer, S.; Paul, M.; Schwalbe, M.; Peuntinger, K.; Guldi, D.M.; Guthmuller, J.; Popp, J.; Gräfe, S.; et al. Tuning of photocatalytic activity by creating a tridentate coordination sphere for palladium. Dalton Trans. 2014, 43, 11676–11686. [Google Scholar] [CrossRef]
- Kaufhold, S.; Imanbaew, D.; Riehn, C.; Rau, S. Rational in situ tuning of a supramolecular photocatalyst for hydrogen evolution. Sustain. Energy Fuels 2017, 1, 2066–2070. [Google Scholar] [CrossRef]
- Das, N.; Bindra, G.S.; Paul, A.; Vos, J.G.; Schulz, M.; Pryce, M.T. Enhancing photocatalytic hydrogen generation: The impact of the peripheral ligands in Ru/Pd and Ru/Pt complexes. Chem. Eur. J. 2017, 23, 5330–5337. [Google Scholar] [CrossRef] [PubMed]
- Stoll, T.; Gennari, M.; Fortage, J.; Castillo, C.E.; Rebarz, M.; Sliwa, M.; Poizat, O.; Odobel, F.; Deronzier, A.; Collomb, M.-N. An efficient RuII–RhIII–RuII polypyridyl photocatalyst for visible-light-driven hydrogen production in aqueous solution. Angew. Chem. Int. Ed. 2014, 53, 1654–1658. [Google Scholar] [CrossRef] [PubMed]
- Lentz, C.; Schott, O.; Auvray, T.; Hanan, G.S.; Elias, B. Design and photophysical studies of iridium(III)–cobalt(III) dyads and their application for dihydrogen photo-evolution. Dalton Trans. 2019, 48, 15567–15576. [Google Scholar] [CrossRef]
- Zedler, L.; Wintergerst, P.; Mengele, A.K.; Müller, C.; Li, C.; Dietzek-Ivanšić, B.; Rau, S. Outpacing conventional nicotinamide hydrogenation catalysis by a strongly communicating heterodinuclear photocatalyst. Nat. Commun. 2022, 13, 2538. [Google Scholar] [CrossRef]
- Kimura, E.; Wada, S.; Shionoya, M.; Takahashi, T.; Litaka, Y. A novel cyclam–nickel(II) complex appended with a tris-(2,2′-bipyridine) ruthenium(II) complex (cyclam = 1,4,8,11-tetra-azacyclotetradecane). J. Chem. Soc. Chem. Commun. 1990, 5, 397–398. [Google Scholar] [CrossRef]
- Kimura, E.; Bu, X.; Shionoya, M.; Wada, S.; Maruyama, S. A new nickel(II) cyclam (cyclam = 1,4,8,11-tetraazacyclotetradecane) complex covalently attached to tris(1,10-phenanthroline)ruthenium(2+). A new candidate for the catalytic photoreduction of carbon dioxide. Inorg. Chem. 1992, 31, 4542–4546. [Google Scholar] [CrossRef]
- Kimura, E.; Wada, S.; Shionoya, M.; Okazaki, Y. New series of multifunctionalized nickel(II)-cyclam (cyclam = 1,4,8,11-tetraazacyclotetradecane) complexes. application to the photoreduction of carbon dioxide. Inorg. Chem. 1994, 33, 770–778. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Himeda, Y.; Hirose, T.; Sugihara, H.; Kasuga, K. Synthesis and photochemical properties of ruthenium–cobalt and ruthenium–nickel dinuclear complexes. Bull. Chem. Soc. Jpn. 1999, 72, 725–731. [Google Scholar] [CrossRef]
- Gholamkhass, B.; Mametsuka, H.; Koike, K.; Tanabe, T.; Furue, M.; Ishitani, O. Architecture of supramolecular metal complexes for photocatalytic CO2 reduction: ruthenium−rhenium bi- and tetranuclear complexes. Inorg. Chem. 2005, 44, 2326–2336. [Google Scholar] [CrossRef]
- Koike, K.; Naito, S.; Sato, S.; Tamaki, Y.; Ishitani, O. Architecture of supramolecular metal complexes for photocatalytic CO2 reduction: III: Effects of length of alkyl chain connecting photosensitizer to catalyst. J. Photochem. Photobiol. A Chem. 2009, 207, 109–114. [Google Scholar] [CrossRef]
- Bian, Z.-Y.; Chi, S.-M.; Li, L.; Fu, W. Conjugation effect of the bridging ligand on the CO2 reduction properties in difunctional photocatalysts. Dalton Trans. 2010, 39, 7884–7887. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Koike, K.; Morimoto, T.; Ishitani, O. Substantial improvement in the efficiency and durability of a photocatalyst for carbon dioxide reduction using a benzoimidazole derivative as an electron donor. J. Catal. 2013, 304, 22–28. [Google Scholar] [CrossRef]
- Kato, E.; Takeda, H.; Koike, K.; Ohkubo, K.; Ishitani, O. Ru(II)–Re(I) binuclear photocatalysts connected by –CH2XCH2– (X = O, S, CH2) for CO2 reduction. Chem. Sci. 2015, 6, 3003–3012. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Ohkubo, K.; Saito, D.; Yatsu, T.; Tamaki, Y.; Tanaka, S.I.; Koike, K.; Onda, K.; Ishitani, O. Kinetics and mechanism of intramolecular electron transfer in Ru(II)–Re(I) supramolecular CO2–reduction photocatalysts: Effects of bridging ligands. Inorg. Chem. 2019, 58, 11480–11492. [Google Scholar] [CrossRef]
- Tamaki, Y.; Watanabe, K.; Koike, K.; Inoue, H.; Morimoto, T.; Ishitani, O. Development of highly efficient supramolecular CO2 reduction photocatalysts with high turnover frequency and durability. Faraday Discuss. 2012, 155, 115–127. [Google Scholar] [CrossRef]
- Ohkubo, K.; Yamazaki, Y.; Nakashima, T.; Tamaki, Y.; Koike, K.; Ishitani, O. Photocatalyses of Ru(II)–Re(I) binuclear complexes connected through two ethylene chains for CO2 reduction. J. Catal. 2016, 343, 278–289. [Google Scholar] [CrossRef]
- Brown, C.M.; Auvray, T.; DeLuca, E.E.; Ezhova, M.B.; Hanan, G.S.; Wolf, M.O. Controlling photocatalytic reduction of CO2 in Ru(II)/Re(I) dyads via linker oxidation state. Chem. Commun. 2020, 56, 10750–10753. [Google Scholar] [CrossRef]
- Gotico, P.; Tran, T.-T.; Baron, A.; Vauzeilles, B.; Lefumeux, C.; Ha-Thi, M.-H.; Pino, T.; Halime, Z.; Quaranta, A.; Leibl, W.; et al. Tracking charge accumulation in a functional triazole-linked ruthenium-rhenium dyad towards photocatalytic carbon dioxide reduction. ChemPhotoChem 2021, 5, 654–664. [Google Scholar] [CrossRef]
- Cerpentier, F.J.R.; Karlsson, J.; Lalrempuia, R.; Brandon, M.P.; Sazanovich, I.V.; Greetham, G.M.; Gibson, E.A.; Pryce, M.T. Ruthenium assemblies for CO2 reduction and H2 generation: Time resolved infrared spectroscopy, spectroelectrochemistry and a photocatalysis study in solution and on NiO. Front. Chem. 2021, 9, 795877. [Google Scholar] [CrossRef]
- Kuttassery, F.; Kumagai, H.; Kamata, R.; Ebato, Y.; Higashi, M.; Suzuki, H.; Abe, R.; Ishitani, O. Supramolecular photocatalysts fixed on the inside of the polypyrrole layer in dye sensitized molecular photocathodes: Application to photocatalytic CO2 reduction coupled with water oxidation. Chem. Sci. 2021, 12, 13216–13232. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.S.; Thomas, C.; Kränzlein, M.; Pehl, T.M.; Rieger, B. Macromolecular rhenium–ruthenium complexes for photocatalytic CO2 conversion: From catalytic Lewis pair polymerization to well-defined poly(vinyl bipyridine)–metal complexes. Macromolecules 2022, 55, 7039–7048. [Google Scholar] [CrossRef]
- Fabry, D.C.; Koizumi, H.; Ghosh, D.; Yamazaki, Y.; Takeda, H.; Tamaki, Y.; Ishitani, O. A Ru(II)–Mn(I) supramolecular photocatalyst for CO2 reduction. Organometallics 2020, 39, 1511–1518. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Takeda, H.; Ohashi, K.; Asatani, T.; Kosumi, D.; Hashimoto, H.; Ishitani, O.; Tamiaki, H. Photochemical reduction of CO2 with red light using synthetic chlorophyll–rhenium bipyridine dyad. Chem. Lett. 2014, 43, 1383–1385. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Fujisawa, Y.; Satake, A. Photocatalytic CO2 reduction mediated by electron transfer via the excited triplet state of Zn(II) porphyrin. J. Am. Chem. Soc. 2020, 142, 705–709. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Satake, A. Photocatalytic CO2 reductions catalyzed by meso-(1,10-phenanthrolin-2-yl)-porphyrins having a rhenium(I) tricarbonyl complex. Chem. Eur. J. 2020, 26, 16365–16373. [Google Scholar] [CrossRef]
- Lang, P.; Pfrunder, M.; Quach, G.; Braun-Cula, B.; Moore, E.G.; Schwalbe, M. Sensitized photochemical CO2 reduction by hetero-Pacman compounds linking a ReI tricarbonyl with a porphyrin unit. Chem. Eur. J. 2019, 25, 4509–4519. [Google Scholar] [CrossRef] [PubMed]
- Matlachowski, C.; Braun, B.; Tschierlei, S.; Schwalbe, M. Photochemical CO2 reduction catalyzed by phenanthroline extended tetramesityl porphyrin complexes linked with a rhenium(I) tricarbonyl unit. Inorg. Chem. 2015, 54, 10351–10360. [Google Scholar] [CrossRef]
- Schneider, J.; Vuong, K.Q.; Calladine, J.A.; Sun, X.-Z.; Whitwood, A.C.; George, M.W.; Perutz, R.N. Photochemistry and photophysics of a Pd(II) metalloporphyrin: Re(I) tricarbonyl bipyridine molecular dyad and its activity toward the photoreduction of CO2 to CO. Inorg. Chem. 2011, 50, 11877–11889. [Google Scholar] [CrossRef] [PubMed]
- Windle, C.D.; Câmpian, M.V.; Duhme-Klair, A.-K.; Gibson, E.A.; Perutz, R.N.; Schneider, J. CO2 photoreduction with long-wavelength light: Dyads and monomers of zinc porphyrin and rhenium bipyridine. Chem. Commun. 2012, 48, 8189–8191. [Google Scholar] [CrossRef]
- Windle, C.D.; George, M.W.; Perutz, R.N.; Summers, P.A.; Sun, X.Z.; Whitwood, A.C. Comparison of rhenium–porphyrin dyads for CO2 photoreduction: Photocatalytic studies and charge separation dynamics studied by time-resolved IR spectroscopy. Chem. Sci. 2015, 6, 6847–6864. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, A.; Edure, S.; Yatsuda, S.; Akita, M. Highly selective photo-catalytic dimerization of α-methylstyrene by a novel palladium complex with photosensitizing ruthenium(II) polypyridyl moiety. Chem. Commun. 2005, 43, 5468–5470. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, A.; Yatsuda, S.; Edure, S.; Suzuki, A.; Takahashi, T.; Akita, M. Synthesis of Pd complexes combined with photosensitizing of a ruthenium(II) polypyridyl moiety through a series of substituted bipyrimidine bridges. Substituent effect of the bridging ligand on the photocatalytic dimerization of α-methylstyrene. Inorg. Chem. 2007, 46, 2432–2445. [Google Scholar] [CrossRef] [PubMed]
- Nitadori, H.; Takahashi, T.; Inagaki, A.; Akita, M. Enhanced photocatalytic activity of α-methylstyrene oligomerization through effective metal-to-ligand charge-transfer localization on the bridging ligand. Inorg. Chem. 2012, 51, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Araki, M.; Inagaki, A.; Akita, M. Syntheses, photophysical properties, and reactivities of novel bichromophoric Pd complexes composed of Ru(II)–polypyridyl and naphthyl moieties. Dalton Trans. 2013, 42, 6989–7001. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Inagaki, A.; Akita, M.; Halet, J.-F.; Costuas, K. Revelation of the photoactive species in the photocatalytic dimerization of α-methylstyrene by a dinuclear ruthenium–palladium complex. Inorg. Chem. 2013, 52, 8030–8039. [Google Scholar] [CrossRef]
- Kikuchi, S.; Saito, K.; Akita, M.; Inagaki, A. Nonradical light-controlled polymerization of styrene and vinyl ethers catalyzed by an iridium-palladium photocatalyst. Organometallics 2018, 37, 359–366. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nomura, K.; Inagaki, A. Cu–Pd dinuclear complexes with earth-abundant Cu photosensitizer: Synthesis and photopolymerization. Organometallics 2020, 39, 2464–2469. [Google Scholar] [CrossRef]
- Armaroli, N. Photoactive mono- and polynuclear Cu(I)–phenanthrolines. A viable alternative to Ru(II)–polypyridines? Chem. Soc. Rev. 2001, 30, 113–124. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef]
- Buchwald, S.L. Cross coupling. Acc. Chem. Res. 2008, 41, 1439. [Google Scholar] [CrossRef] [PubMed]
- Johansson Seechurn, C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Devendar, P.; Qu, R.-Y.; Kang, W.-M.; He, B.; Yang, G.-F. Palladium-Catalyzed Cross-Coupling Reactions: A Powerful Tool for the Synthesis of Agrochemicals. J. Agric. Food. Chem. 2018, 66, 8914–8934. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Nagai, H.; Akita, M. Photo-activation of Pd-catalyzed Sonogashira coupling using a Ru/bipyridine complex as energy transfer agent. Dalton Trans. 2007, 8, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, K.C.; Ebukuyo, P.O.; Dhahir, Y.J.; Wheeler, K.; He, H. A BODIPY-functionalized PdII photoredox catalyst for Sonogashira C–C cross-coupling reactions. Chem. Commun. 2019, 55, 4973–4976. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kawashima, M.; Yamashita, H. Visible-light-enhanced Suzuki–Miyaura coupling reaction by cooperative photocatalysis with an Ru–Pd bimetallic complex. Chem. Commun. 2014, 50, 14501–14503. [Google Scholar] [CrossRef]
- Yao, S.Y.; Cao, M.L.; Zhang, X.L. Photoaccelerated energy transfer catalysis of the Suzuki-Miyaura coupling through ligand regulation on Ir(III)-Pd(II) bimetallic complexes. RSC Adv. 2020, 10, 42874–42882. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Photoaccelerated Suzuki–Miyaura and Sonogashira coupling reactions catalyzed by an Ir-Pd binuclear complex. Mol. Catal. 2022, 522, 112232. [Google Scholar] [CrossRef]
- Li, M.; Chia, X.L.; Zhu, Y. Tethered photocatalyst-directed palladium-catalysed C–H allenylation of N-aryl tetrahydroisoquinolines. Chem. Commun. 2022, 58, 4719–4722. [Google Scholar] [CrossRef]
- Herrero, C.; Quaranta, A.; Fallahpour, R.-A.; Leibl, W.; Aukauloo, A. Identification of the Different Mechanisms of Activation of a [RuII(tpy)(bpy)(OH2)]2+ Catalyst by Modified Ruthenium Sensitizers in Supramolecular Complexes. J. Phys. Chem. C 2013, 117, 9605–9612. [Google Scholar] [CrossRef]
- Herrero, C.; Quaranta, A.; Leibl, W.; Rutherford, A.W.; Aukauloo, A. Artificial photosynthetic systems. Using light and water to provide electrons and protons for the synthesis of a fuel. Energy Environ. Sci. 2011, 4, 2353–2365. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; Alsaleh, A.Z.; Charalambidis, G.; Coutsolelos, A.G.; D’Souza, F. A covalently linked nickel(II) porphyrin–ruthenium(II) tris(bipyridyl) dyad for efficient photocatalytic water oxidation. Chem. Commun. 2022, 58, 12078–12081. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Fu, W.-F. Insight into highly selective photocatalytic oxidation of alcohols by a new trinuclear ruthenium complex with visible light. Dalton Trans. 2014, 43, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Herrero, C.; Quaranta, A.; Sircoglou, M.; Sénéchal-David, K.; Baron, A.; Marín, I.M.; Buron, C.; Baltaze, J.-P.; Leibl, W.; Aukauloo, A.; et al. Successive light-induced two electron transfers in a Ru–Fe supramolecular assembly: From Ru–Fe(II)–OH2 to Ru–Fe(IV)–oxo. Chem. Sci. 2015, 6, 2323–2327. [Google Scholar] [CrossRef]
- Skolia, E.; Gkizis, P.L.; Kokotos, C.G. Aerobic photocatalysis: Oxidation of sulfides to sulfoxides. ChemPlusChem 2022, 87, e202200008. [Google Scholar] [CrossRef]
- Baciocchi, E.; Giacco, T.D.; Elisei, F.; Gerini, M.F.; Guerra, M.; Lapi, A.; Liberali, P. Electron transfer and singlet oxygen mechanisms in the photooxygenation of dibutyl sulfide and thioanisole in MeCN Sensitized by N-methylquinolinium tetrafluoborate and 9,10-dicyanoanthracene. The probable involvement of a thiadioxirane intermediate in electron transfer photooxygenations. J. Am. Chem. Soc. 2003, 125, 16444–16454. [Google Scholar] [CrossRef]
- Clennan, E.L. Persulfoxide: Key intermediate in reactions of singlet oxygen with sulfides. Acc. Chem. Res. 2001, 34, 875–884. [Google Scholar] [CrossRef]
- Iali, W.; Lanoe, P.-H.; Torelli, S.; Jouvenot, D.; Loiseau, F.; Lebrun, C.; Hamelin, O.; Ménage, S. A ruthenium(II)–copper(II) dyad for the photocatalytic oxygenation of organic substrates mediated by dioxygen activation. Angew. Chem. Int. Ed. 2015, 54, 8415–8419. [Google Scholar] [CrossRef]
- Chao, D.; Zhao, M. Robust Cooperative photo-oxidation of sulfides without sacrificial reagent under air using a dinuclear RuII–CuII assembly. ChemSusChem 2017, 10, 3358–3362. [Google Scholar] [CrossRef]
- Ananikov, V.P. Nickel: The “spirited horse” of transition metal catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- Thoke, M.B.; Sun, G.-J.; Borse, R.A.; Lin, P.; Lin, S.-X. Unimolecular cooperative metallaphotocatalysis with conjugately bridged Ir–Ni complexes and its applications in organic coupling reactions. Org. Chem. Front. 2022, 9, 1797–1807. [Google Scholar] [CrossRef]
- Kumar, P.; Joshi, C.; Srivastava, A.K.; Gupta, P.; Boukherroub, R.; Jain, S.L. Visible light assisted photocatalytic [3+2] azide–alkyne “Click” reaction for the synthesis of 1,4-substituted 1,2,3-triazoles using a novel bimetallic Ru–Mn complex. ACS Sustain. Chem. Eng. 2016, 4, 69–75. [Google Scholar] [CrossRef]
- Bayer, L.; Birenheide, B.S.; Krämer, F.; Lebedkin, S.; Breher, F. Heterobimetallic gold/ruthenium complexes synthesized via post-functionalization and applied in dual photoredox gold catalysis. Chem. Eur. J. 2022, 28, e202201856. [Google Scholar] [CrossRef] [PubMed]











































| Entry | R | R’ | Conditions | Yield, % | Reference |
|---|---|---|---|---|---|
| 1 | H | H | RuPd-23/PPh3, (1/2 mol.%) K2CO3, EtOH, r.t., visible light | 10 | [97] |
| 2 | H | H | RuPd-23/PPh3, (1/2 mol.%) K2CO3, EtOH, r.t., dark | 6 | [97] |
| 3 | H | H | Ru(bpy)2(bpm)2+/ Pd(bpy)Cl2/PPh3 (1/1/2 mol.%) K2CO3, EtOH, r.t., visible light | 6 | [97] |
| 4 | H | H | Pd(bpy)Cl2/PPh3 (1/2 mol.%) K2CO3, EtOH, r.t., visible light | 5 | [97] |
| 5 | Me | H | RuPd-23/PPh3, (2.5/5 mol.%) Cs2CO3, DCM-EtOH, r.t., blue LED | 80 | [98] |
| 6 | Me | H | IrPd-2/PPh3, (2.5/5 mol.%) Cs2CO3, DCM-EtOH, r.t., blue LED | 86 | [98] |
| 7 | Me | H | IrPd-3/PPh3, (2.5/5 mol.%) Cs2CO3, DCM-EtOH, r.t., blue LED | 54 | [98] |
| 8 | Me | H | IrPd-4/PPh3, (2.5/5 mol.%)) Cs2CO3, DCM-EtOH, r.t., blue LED | 93 | [98] |
| 9 | Me | H | IrPd-4/PPh3, (2.5/5 mol.%)) Cs2CO3, DCM-EtOH, r.t., dark | 40 | [98] |
| 10 | Me | H | Ir(pq)2(bpy)+/Pd(bpm)Cl2/ PPh3 (2.5/2.5/5 mol.%) Cs2CO3, DCM-EtOH, r.t., blue LED | 75 | [98] |
| 11 | MeC(O) | Me | IrPd-5/PPh3, (1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 99 | [99] |
| 12 | MeC(O) | Me | Ir(ppy)2L/[Pd(ppy)Cl]2/PPh3 (1/1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 35 | [99] |
| 13 | Me | Me | IrPd-5/PPh3, (1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 98 | [99] |
| 14 | CN | Me | IrPd-5/PPh3, (1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 98 | [99] |
| 15 | OMe | Me | IrPd-5/PPh3, (1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 78 | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionova, V.A.; Abel, A.S.; Averin, A.D.; Beletskaya, I.P. Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis. Catalysts 2023, 13, 768. https://doi.org/10.3390/catal13040768
Ionova VA, Abel AS, Averin AD, Beletskaya IP. Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis. Catalysts. 2023; 13(4):768. https://doi.org/10.3390/catal13040768
Chicago/Turabian StyleIonova, Violetta A., Anton S. Abel, Alexei D. Averin, and Irina P. Beletskaya. 2023. "Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis" Catalysts 13, no. 4: 768. https://doi.org/10.3390/catal13040768
APA StyleIonova, V. A., Abel, A. S., Averin, A. D., & Beletskaya, I. P. (2023). Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis. Catalysts, 13(4), 768. https://doi.org/10.3390/catal13040768