In Situ Growth of Ti3C2/UiO-66-NH2 Composites for Photoreduction of Cr(VI)
Abstract
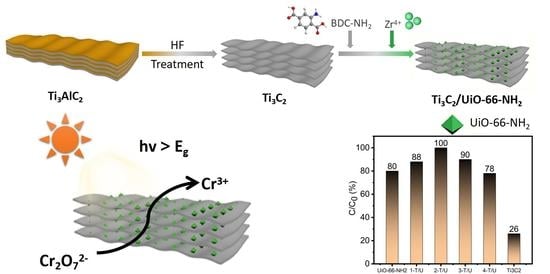
:1. Introduction
2. Results and Discussion
2.1. Structural Characterizations
2.2. Photoelectrochemical Characterizations of Photocatalysts
2.3. Photocatalytic Performance of Photocatalysts
3. Materials and Methods
3.1. Chemical Regents
3.2. Synthesis of Accordion-Like Ti3C2 MXene
3.3. Synthesis of UiO-66-NH2
3.4. Synthesis of Ti3C2/UiO-66-NH2
3.5. Material Characterizations
3.6. Photoelectrochemical Characterizations
3.7. Photocatalytic Activity
3.8. Standard Curve Diagram
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wakeel, A.; Xu, M. Chromium morpho-phytotoxicity. Plants 2020, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, V.; Kim, K.-H.; Kwon, E.E.; Younis, S.A. Metal-organic frameworks for photocatalytic detoxification of chromium and uranium in water. Coord. Chem. Rev. 2021, 447, 214148. [Google Scholar] [CrossRef]
- Chen, D.D.; Yi, X.H.; Zhao, C.; Fu, H.; Wang, P.; Wang, C.C. Polyaniline modified MIL-100(Fe) for enhanced photocatalytic Cr(VI) reduction and tetracycline degradation under white light. Chemosphere 2020, 245, 125659. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qian, X.; Wei, Q.; Chen, Z.; Chen, J.; Wang, W.; Chen, X.; Gao, J.; Liu, Y.; Xie, L. Construction of hydrostable cesium lead bromide-titania for visible-light degradation of tetracycline hydrochloride in water. J. Clean. Prod. 2022, 365, 132830. [Google Scholar] [CrossRef]
- Gao, J.; Qian, X.; Wei, Q.; Chen, Z.; Liu, C.; Wang, W.; Chen, J.; Chen, X.; Liu, Y.; Wei, G. Construction of core-shell cesium lead bromide-silica by precipitation coating method with applications in aqueous photocatalysis. J. Colloid Interface Sci. 2022, 623, 974–984. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhao, W.; Liu, C.; Qian, X.; Zhang, M.; Wei, G.; Khan, E.; Hau Ng, Y.; Sik Ok, Y. Recent advances in photodegradation of antibiotic residues in water. Chem. Eng. J. 2021, 405, 126806. [Google Scholar] [CrossRef]
- Xiong, Q.; Chen, Y.; Xu, T.; Zhu, Z.; Chen, W.; Lu, W. Highly efficient purification of emerging pollutants and bacteria in natural water by g-C3N4-sheltered fibers containing TiO2. Appl. Surf. Sci. 2021, 559, 149839. [Google Scholar] [CrossRef]
- Wang, T.; Dai, Z.; Kang, J.; Fu, F.; Zhang, T.; Wang, S. A TiO2 nanocomposite hydrogel for hydroponic plants in efficient water improvement. Mater. Chem. Phys. 2018, 215, 242–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Ren, X.; Luo, S.; Huang, H.; Chen, R.; Shao, S.; Liu, D.; Gao, J.; Gui, J.; et al. Building rapid charge transfer channel via engineering Ni coordinated flexible polymer for efficient solar hydrogen evolution. Chem. Eng. J. 2023, 456, 141032. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, Y.; Zhu, Y.; Fu, Y. Improved visible-light photocatalytic activity of sodium tantalum oxide via biomass-derived silk fibroin doping. Text. Res. J. 2018, 89, 1332–1339. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, G.; Li, L.; Chen, H.; Zhou, H.; Yao, J.; Kong, X.; Chen, W. N-Doped carbon hybrid conjugates as vectors for photocatalytic CS2 production. Mater. Res. Express 2015, 2, 045603. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Y.; Cui, J.; Wang, S.; Wang, T. Carbon nanotubes-dispersed TiO2 nanoparticles with their enhanced photocatalytic activity. Mater. Res. Bull. 2014, 59, 278–282. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Li, J.; Zhang, G. Boosting interfacial charge separation of Ba5Nb4O15/g-C3N4 photocatalysts by 2D/2D nanojunction towards efficient visible-light driven H2 generation. Appl. Catal. B Environ. 2020, 263, 117730. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Wang, K.; Zhang, G.; Li, Y.; Wu, X. Low boiling point solvent mediated strategy to synthesize functionalized monolayer carbon nitride for superior photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 260, 118181. [Google Scholar] [CrossRef]
- Jamshaid, M.; Nazir, M.A.; Najam, T.; Shah, S.S.A.; Khan, H.M.; Rehman, A.U. Facile synthesis of Yb3+-Zn2+ substituted M type hexaferrites: Structural, electric and photocatalytic properties under visible light for methylene blue removal. Chem. Phys. Lett. 2022, 805, 139939. [Google Scholar] [CrossRef]
- Jamshaid, M.; Khan, M.I.; Fernandez, J.; Shanableh, A.; Hussain, T.; Rehman, A.U. Synthesis of Ti4+ doped Ca-BiFO3 for the enhanced photodegradation of moxifloxacin. New J. Chem. 2022, 46, 19848–19856. [Google Scholar] [CrossRef]
- Jamshaid, M.; Khan, H.M.; Nazir, M.A.; Wattoo, M.A.; Shahzad, K.; Malik, M.; Rehman, A.-U. A novel bentonite–cobalt doped bismuth ferrite nanoparticles with boosted visible light induced photodegradation of methyl orange: Synthesis, characterization and analysis of physiochemical changes. Int. J. Environ. Anal. Chem. 2022, 1–16. [Google Scholar] [CrossRef]
- Wang, C.C.; Ren, X.; Wang, P.; Chang, C. The state of the art review on photocatalytic Cr(VI) reduction over MOFs-based photocatalysts: From batch experiment to continuous operation. Chemosphere 2022, 303, 134949. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Y.; Zhu, X.; Fang, J.; Xu, W.; Hu, X.; Li, R.; Yao, L.; Qin, J.; Fang, Z. Semiconductor heterojunctions for photocatalytic hydrogen production and Cr(VI) Reduction: A review. Mater. Res. Bull. 2022, 147, 111636. [Google Scholar] [CrossRef]
- Jafarzadeh, M. Recent progress in the development of MOF-based photocatalysts for the photoreduction of Cr(VI). ACS Appl. Mater. Interfaces 2022, 14, 24993–25024. [Google Scholar] [CrossRef]
- Liu, W.; Guo, Z.; Jin, Z.; Chen, D.; Lu, T.; Jia, P.; Xing, H. Visible-light-driven sonophotocatalysis for enhanced Cr(VI) reduction over mixed-linker zirconium-porphyrin MOFs. Catal. Sci. Technol. 2022, 12, 2176–2183. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, C.; Li, H.; Huang, Y.; Cao, R. Graphene quantum dots supported on Fe-based metal-organic frameworks for efficient photocatalytic CO2 reduction. Acta Chim. Sin. 2022, 80, 22–28. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-functionalized titanium based metal-organic framework for photocatalytic hydrogen production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef]
- Lei, Z.; Tang, Q.; Ju, Y.; Lin, Y.; Bai, X.; Luo, H.; Tong, Z. Block copolymer@ZIF-8 nanocomposites as a pH-responsive multi-steps release system for controlled drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cai, H.; Li, X.; Zhang, J.; Zhang, D.; Yang, Y.; Xiong, J. Construction of hierarchical NiCo2O4@Ni-MOF hybrid arrays on carbon cloth as superior battery-type electrodes for flexible solid-state hybrid supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 37675–37684. [Google Scholar] [CrossRef]
- Khan, U.; Nairan, A.; Gao, J.K.; Zhang, Q.C. Current progress in 2D metal-organic frameworks for electrocatalysis. Small Struct. 2022, 2200109. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, H.; Liu, P.; Chen, J.; Xu, H.; Alshahrani, T.; Li, L.; Chen, B.; Gao, J. Robust microporous hydrogen−bonded organic framework for highly selective purification of methane from natural gas. Microporous Mesoporous Mater. 2023, 352, 112495. [Google Scholar] [CrossRef]
- Li, Y.W.; Wu, Y.H.; Li, T.T.; Lu, M.T.; Chen, Y.; Cui, Y.J.; Gao, J.K.; Qian, G.D. Tuning the electronic structure of a metal-organic framework for an efficient oxygen evolution reaction by introducing minor atomically dispersed ruthenium. Carbon Energy 2023, 5, e265. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Wang, L.; Sun, P.; Wang, P.; Yao, Z.; Yang, Y. Coupling bimetallic NiMn-MOF nanosheets on NiCo2O4 nanowire arrays with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2022, 149, 111707. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, S.; Wang, J.; Tai, M.; Wang, X.; Ding, Y.; Jin, D.; Wang, L. Highly improved photoluminescence properties of novel ternary Eu(cpioa)phen metal–organic frameworks. Funct. Mater. Lett. 2022, 15, 2251026. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo)Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196. [Google Scholar] [CrossRef]
- Fan, L.; Yu, Q.; Chen, J.; Khan, U.; Wang, X.; Gao, J. Achievements and perspectives in metal–organic framework-based materials for photocatalytic nitrogen reduction. Catalysts 2022, 12, 1005. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, L.; Li, D.; Ye, J. Recent progress on exploring stable metal–organic frameworks for photocatalytic solar fuel production. Sol. RRL 2020, 4, 1900547. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.S.; Liu, T.F.; Ye, J. Titanium-based MOF materials: From crystal engineering to photocatalysis. Small Methods 2020, 4, 2000486. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.-S.; Philo, D.; Ichihara, F.; Song, H.; Li, Y.; Li, D.; Qiu, T.; Wang, S.; Ye, J. Toward visible-light-assisted photocatalytic nitrogen fixation: A titanium metal organic framework with functionalized ligands. Appl. Catal. B Environ. 2020, 267, 118686. [Google Scholar] [CrossRef]
- Wang, C.-C.; Du, X.-D.; Li, J.; Guo, X.-X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B Environ. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Shen, L.; Liang, R.; Luo, M.; Jing, F.; Wu, L. Electronic effects of ligand substitution on metal-organic framework photocatalysts: The case study of UiO-66. Phys. Chem. Chem. Phys. 2015, 17, 117–121. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Jiang, L.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J. Hazard Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef]
- Wang, X.-S.; Chen, C.-H.; Ichihara, F.; Oshikiri, M.; Liang, J.; Li, L.; Li, Y.; Song, H.; Wang, S.; Zhang, T.; et al. Integration of adsorption and photosensitivity capabilities into a cationic multivariate metal-organic framework for enhanced visible-light photoreduction reaction. Appl. Catal. B Environ. 2019, 253, 323–330. [Google Scholar] [CrossRef]
- Lamiel, C.; Hussain, I.; Warner, J.H.; Zhang, K. Beyond Ti-based MXenes: A review of emerging non-Ti based metal-MXene structure, properties, and applications. Mater. Today 2023, 63, 313–338. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Solangi, N.H.; Karri, R.R.; Mazari, S.A.; Mubarak, N.M.; Jatoi, A.S.; Malafaia, G.; Azad, A.K. MXene as emerging material for photocatalytic degradation of environmental pollutants. Coord. Chem. Rev. 2023, 477, 214965. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, R.; Qin, J.; Hu, H.; Fan, X.; Cao, X.; Wang, D. MIL-100(Fe)/Ti3C2 MXene as a schottky catalyst with enhanced photocatalytic oxidation for nitrogen fixation activities. ACS Appl. Mater. Interfaces 2019, 11, 44249–44262. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Sasaki, T.; Yunoki, S. Electronic properties and applications of MXenes: A theoretical review. J. Mater. Chem. C 2017, 5, 2488–2503. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Chen, Y.; Hou, B.; Zhang, N.; Chen, B.; Yang, N.; Chen, K.; Li, J.; An, L. Fabrication of layered Ti3C2 with an accordion-like structure as a potential cathode material for high performance lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 7870–7876. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colon, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.-F.; Xi, F.-G.; Gao, E.-Q. Amino-functionalized Zr(IV) metal–organic framework as bifunctional acid–base catalyst for Knoevenagel condensation. J. Mol. Catal. A Chem. 2014, 390, 198–205. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Z.; Shao, L.; Li, X.; Zhu, M.; Liu, Y.; Feng, X.; Zhu, X. A novel strategy for enhancing the performance of membranes for dyes separation: Embedding PAA@UiO-66-NH2 between graphene oxide sheets. Chem. Eng. J. 2021, 403, 126281. [Google Scholar] [CrossRef]
- Zahid, M.; Zhang, D.; Xu, X.; Pan, M.; Ul Haq, M.H.; Reda, A.T.; Xu, W. Barbituric and thiobarbituric acid-based UiO-66-NH2 adsorbents for iodine gas capture: Characterization, efficiency and mechanisms. J. Hazard Mater. 2021, 416, 125835. [Google Scholar] [CrossRef]
- Lin, K.A.; Liu, Y.T.; Chen, S.Y. Adsorption of fluoride to UiO-66-NH2 in water: Stability, kinetic, isotherm and thermodynamic studies. J. Colloid Interface Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, W.X.; Han, Y.; Luo, X.L.; Tang, W.Z.; Yue, T.L.; Li, Z.H. A straightforward strategy to synthesize supramolecular amorphous zirconium metal-organic gel for efficient Pb(II) removal. Chem. Eng. J. 2021, 407, 126744. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.K.; Lee, S.C. Immobilization of polymeric g-C3N4 on structured ceramic foam for efficient visible light photocatalytic air purification with real indoor illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.T.; Ma, T.Y.; Du, A.; Qiao, S.Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Li, Y.; Jiang, L.; Zhang, G. Novel BiSbO4/BiOBr nanoarchitecture with enhanced visible-light driven photocatalytic performance: Oxygen-induced pathway of activation and mechanism unveiling. Appl. Surf. Sci. 2019, 498, 143850. [Google Scholar] [CrossRef]
- Long, R.; Yu, Z.; Tan, Q.; Feng, X.; Zhu, X.; Li, X.; Wang, P. Ti3C2 MXene/NH2-MIL-88B(Fe): Research on the adsorption kinetics and photocatalytic performance of an efficient integrated photocatalytic adsorbent. Appl. Surf. Sci. 2021, 570, 151244. [Google Scholar] [CrossRef]
- Yi, X.-H.; Ma, S.-Q.; Du, X.-D.; Zhao, C.; Fu, H.; Wang, P.; Wang, C.-C. The facile fabrication of 2D/3D Z-scheme g-C3N4/UiO-66 heterojunction with enhanced photocatalytic Cr(VI) reduction performance under white light. Chem. Eng. J. 2019, 375, 121944. [Google Scholar] [CrossRef]
- Du, X.; Yi, X.; Wang, P.; Deng, J.; Wang, C.-C. Enhanced photocatalytic Cr(VI) reduction and diclofenac sodium degradation under simulated sunlight irradiation over MIL-100(Fe)/g-C3N4 heterojunctions. Chin. J. Catal. 2019, 40, 70–79. [Google Scholar] [CrossRef]
- Wang, J.-W.; Qiu, F.-G.; Wang, P.; Ge, C.; Wang, C.-C. Boosted bisphenol A and Cr(VI) cleanup over Z-scheme WO3/MIL-100(Fe) composites under visible light. J. Clean. Prod. 2021, 279, 123408. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, X.; Wang, C.C.; Fu, H.; Liu, Y.; Wang, P.; Zhao, C. S-TiO2/UiO-66-NH2 composite for boosted photocatalytic Cr(VI) reduction and bisphenol A degradation under LED visible light. J. Hazard Mater. 2020, 399, 123085. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-C.; Wang, P.; Fu, H.; Zhao, C.; Wang, C.-C. Ternary Ag/Ag3PO4/MIL-125-NH2 Z-scheme heterojunction for boosted photocatalytic Cr(VI) cleanup under visible light. Chin. Chem. Lett. 2020, 31, 2645–2650. [Google Scholar] [CrossRef]
- He, Q.; Fu, Y.; Ge, X.; Al-Enizi, A.M.; Nafady, A.; Wang, Q.; Ma, S. Facile fabrication of Fe-BDC/Fe-2MI heterojunction with boosted photocatalytic activity for Cr(VI) reduction. J. Environ. Chem. Eng. 2021, 9, 105961. [Google Scholar] [CrossRef]
- Chen, D.D.; Yi, X.H.; Ling, L.; Wang, C.C.; Wang, P. Photocatalytic Cr(VI) sequestration and photo-Fenton bisphenol A decomposition over white light responsive PANI/MIL-88A(Fe). Appl. Organomet. Chem. 2020, 34, e5795. [Google Scholar] [CrossRef]
- Wei, X.; Wang, C.C.; Li, Y.; Wang, P.; Wei, Q. The Z-scheme NH2-UiO-66/PTCDA composite for enhanced photocatalytic Cr(VI) reduction under low-power LED visible light. Chemosphere 2021, 280, 130734. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, J.; Chen, X.; Wang, Z.; Ji, H.; Chen, L.; Liu, W.; Wang, C.C. Bifunctional Bi12O17Cl2/MIL-100(Fe) composites toward photocatalytic Cr(VI) sequestration and activation of persulfate for bisphenol A degradation. Sci. Total Environ. 2021, 752, 141901. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Wang, X.; Yu, Q.; Wu, W.; Feng, X.; Kong, D.; Ren, X.; Gao, J. In Situ Growth of Ti3C2/UiO-66-NH2 Composites for Photoreduction of Cr(VI). Catalysts 2023, 13, 876. https://doi.org/10.3390/catal13050876
He H, Wang X, Yu Q, Wu W, Feng X, Kong D, Ren X, Gao J. In Situ Growth of Ti3C2/UiO-66-NH2 Composites for Photoreduction of Cr(VI). Catalysts. 2023; 13(5):876. https://doi.org/10.3390/catal13050876
Chicago/Turabian StyleHe, Huan, Xusheng Wang, Qin Yu, Wenbin Wu, Xinya Feng, Deyu Kong, Xiaohui Ren, and Junkuo Gao. 2023. "In Situ Growth of Ti3C2/UiO-66-NH2 Composites for Photoreduction of Cr(VI)" Catalysts 13, no. 5: 876. https://doi.org/10.3390/catal13050876
APA StyleHe, H., Wang, X., Yu, Q., Wu, W., Feng, X., Kong, D., Ren, X., & Gao, J. (2023). In Situ Growth of Ti3C2/UiO-66-NH2 Composites for Photoreduction of Cr(VI). Catalysts, 13(5), 876. https://doi.org/10.3390/catal13050876