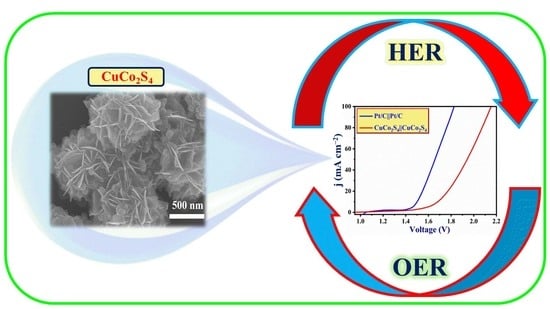
Facile Construction of Three-Dimensional Heterostructured CuCo2S4 Bifunctional Catalyst for Alkaline Water Electrolysis
Abstract
:1. Introduction
2. Results and Discussions
2.1. Structural Characterization of the Catalyst
2.2. Characterization of Electrochemical Performance
2.2.1. Electrochemical Hydrogen Evolution (HER) Performance Analysis
2.2.2. Analysis of Electrochemical Oxygen Evolution (OER) Performance
2.3. Characterization of Overall Water Electrolysis
3. Experimental Section
3.1. Materials
3.2. Synthesis of CuCo2S4 Nanosphere Catalyst
3.3. Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shadidi, B.; Najafi, G.; Yusaf, T. A review of hydrogen as a fuel in internal combustion engines. Energies 2021, 14, 6209. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2016, 16, 16–22. [Google Scholar] [CrossRef]
- Kousik, B.; Moumita, C.; Sanjeev, K.S.; Debabrata, P.; Sang-Jae, K. A critical review on transition metal phosphide based catalyst for electrochemical hydrogen evolution reaction: Gibbs free energy, composition, stability, and true identity of active site. Coord. Chem. Rev. 2023, 478, 214956. [Google Scholar]
- Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, Y.T.; Yao, R.Q.; Wan, W.B.; Ge, X.; Zhang, W.; Wen, Z.; Lang, X.Y.; Zheng, W.T.; Jiang, Q. Spontaneously separated intermetallic Co3Mo from nanoporous copper as versatile electrocatalysts for highly efficient water splitting. Nat. Commun. 2020, 11, 2940. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.; Du, Y.; Xi, S.; Dou, S.; Nsanzimana, J.M.V.; Wang, C.; Xu, Z.J.; Wang, X. Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 2019, 4, 329–338. [Google Scholar] [CrossRef]
- Xiao, P.; Ge, X.; Wang, H.; Liu, Z.; Fisher, A.; Wang, X. Novel molybdenum carbide-tungsten carbide composite nanowires and their electrochemical activation for efficient and stable hydrogen evolution. Adv. Funct. Mater. 2015, 25, 1520–1526. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, X.; Wei, T.; Wang, G.; Zhu, M.; Zhuo, Y.; Cheng, K.; Ye, K.; Zhu, K.; Yan, J.; et al. Hierarchical edge-rich nickel phosphide nanosheet arrays as efficient electrocatalysts toward hydrogen evolution in both alkaline and acidic conditions. ACS Sustain. Chem. Eng. 2019, 7, 7804–7811. [Google Scholar] [CrossRef]
- Ren, C.; Chen, Y.; Du, L.; Wang, Q.; Li, L.; Tian, G. Hierarchical CuCo2S4 nanoflake arrays grown on carbon cloth: A remarkable bifunctional electrocatalyst for overall water splitting. ChemElectroChem 2021, 8, 1134–1140. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Sadhasivam, T.; Oh, T.H. Electrochemically synthesized Cd doped ZnO nanorods and integrated atomic Cd-S bridged Cd:ZnO/CdS heterostructure photoanode for enhanced visible light responsive water oxidation applications. J. Electroanal. Chem. 2023, 934, 117289. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, H.; Qian, X.; He, G.; Chen, H. Sulfur vacancies engineered self-supported Co3S4 nanoflowers as an efficient bifunctional catalyst for electrochemical water splitting. Appl. Catal. B 2023, 322, 122104. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, H.; Rao, D.; Yan, X. Morphology controllable NiCo2S4 nanostructure on carbon cloth for enhanced electrocatalytic water oxidation. J. Alloys Compd. 2022, 897, 163152. [Google Scholar] [CrossRef]
- Li, J.; Xia, Y.; Luo, X.; Mao, T.; Wang, Z.; Hong, Z.; Yue, G. Bi-Phase NiCo2S4-NiS2/CFP Nanocomposites as a Highly Active Catalyst for Oxygen Evolution Reaction. Coatings 2023, 13, 313. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Sekar, K.; Li, R.; Zhu, Y.; Xing, R.; Nakata, K.; Fujishima, A. Hierarchical ZnS@C@MoS2 core-shell nanostructures as efficient hydrogen evolution electrocatalyst for alkaline water electrolysis. Int. J. Hydrogen Energy 2019, 44, 25310–25318. [Google Scholar] [CrossRef]
- Tang, M.; Liu, Y.; Cao, H.; Zheng, Q.; Wei, X.; Lam, K.H.; Lin, D. Cu2S/Ni3S2 ultrathin nanosheets on Ni foam as a highly efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 3013–3021. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Anbarasan, N.; Gunasekaran, A.; Mukilan, M.; Jeganathan, K. Bi2S3 entrenched BiVO4/WO3 multidimensional triadic photoanode for enhanced photoelectrochemical hydrogen evolution applications. Int. J. Hydrogen Energy 2022, 47, 14528–14541. [Google Scholar] [CrossRef]
- Czioska, S.; Wang, J.; Teng, X.; Chen, Z. Hierarchically structured CuCo2S4 nanowire arrays as efficient bifunctional electrocatalyst for overall water splitting. ACS Sustain. Chem. Eng. 2018, 6, 11877–11883. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, K.; Guo, L.; Li, R.; Zaheer, A.; Fu, F.; Xu, B.; Wang, D. In-Situ Construction of 2D/2D CuCo2S4/Bi2WO6 contact heterojunction as a visible-light-driven fenton-like catalyst with highly efficient charge transfer for highly efficient degradation of tetracycline hydrochloride. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127965. [Google Scholar] [CrossRef]
- Liu, T.; Xie, L.; Yang, J.; Kong, R.; Du, G.; Asiri, A.M.; Sun, X.; Chen, L. Self-Standing CoP Nanosheets Array: A Three-Dimensional Bifunctional Catalyst Electrode for Overall Water Splitting in both Neutral and Alkaline Media. ChemElectroChem 2017, 4, 1840–1845. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Yu, Y.; Liu, X.; Shi, Z.; Xing, Y. Self-Assembly of Three-Dimensional Zinc-Doped NiCo2O4 as Efficient Electrocatalysts for Oxygen Evolution Reaction. Chem. Eur. J. 2018, 24, 13002–13008. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Fu, S.; Du, D.; Lin, Y. Facilely Tuning Porous NiCo2O4 Nanosheets with Metal Valence-State Alteration and Abundant Oxygen Vacancies as Robust Electrocatalysts Towards Water Splitting. Chem. Eur. J. 2016, 22, 4000–4007. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Wu, F.; Huang, K.; Hussain, N.; Zu, D.; Wei, H.; Ge, B.; Yao, H.; Liu, L.; Li, H.; et al. Boosting the electrocatalytic water oxidation performance of CoFe2O4 nanoparticles by surface defect engineering. ACS Appl. Mater. Interfaces 2019, 11, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kuai, L.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.P.; Huotari, S.; Yu, M.; Geng, B. Mass-Production of Mesoporous MnCo2O4 Spinels with Manganese (IV)-and Cobalt (II)-Rich Surfaces for Superior Bifunctional Oxygen Electrocatalysis. Angew. Chem. 2017, 129, 15173–15177. [Google Scholar] [CrossRef]
- Du, X.; Lian, W.; Zhang, X. Homogeneous core-shell NiCo2S4 nanorods as flexible electrode for overall water splitting. Int. J. Hydrogen Energy 2018, 43, 20627–20635. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, P.; Yang, F.; Wang, L.; Yin, J.; Ding, J.; Huang, F.; Wang, Y. Effect of FeCo2S4 on electrocatalytic I3- reduction: Theoretical and experimental aspects. Chem. Eng. J. 2021, 424, 130419. [Google Scholar] [CrossRef]
- Sekar, K.; Raji, G.; Chen, S.; Liu, S.; Xing, R. Ultrathin VS2 nanosheets vertically aligned on NiCo2S4@C3N4 hybrid for asymmetric supercapacitor and alkaline hydrogen evolution reaction. Appl. Surf. Sci. 2020, 527, 146856. [Google Scholar] [CrossRef]
- Chauhan, M.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Copper cobalt sulfide nanosheets realizing a promising electrocatalytic oxygen evolution reaction. ACS Catal. 2017, 7, 5871–5879. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, X.; Zhu, C.; Liu, H.; Hou, L. Nickel cobalt sulfide double-shelled hollow nanospheres as superior bifunctional electrocatalysts for photovoltaics and alkaline hydrogen evolution. ACS Appl. Mater. Interfaces 2018, 10, 9379–9389. [Google Scholar] [CrossRef]
- Li, Y.; Yin, J.; An, L.; Lu, M.; Sun, K.; Zhao, Y.Q.; Cheng, F.; Xi, P. Metallic CuCo2S4 nanosheets of atomic thickness as efficient bifunctional electrocatalysts for portable, flexible Zn-air batteries. Nanoscale 2018, 10, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.Q.; Hu, F.C.; Tian, F.Y.; Hou, D.F.; Li, D.S. Equilibrium and kinetic studies on MB adsorption by ultrathin 2D MoS2 nanosheets. RSC Adv. 2016, 6, 11631–11636. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOC sensors. J. Mater. Chem. A, 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Z.; Duan, Y.; Shen, X.; Che, S. Optically active chiral Ag nanowires. Sci. China Mater. 2015, 58, 441–446. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Yin, Y.; Fan, L.; Zhang, N.; Sun, K. In situ synthesis of CuCo2S4@N/S-doped graphene composites with pseudocapacitive properties for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 11708–11714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Zhang, Q.; Li, Y.; Gu, L.; Dai, Z.; Liu, S.; Lan, Y.Q.; Han, M.; Bao, J. Two-dimensional nanostructures of non-layered ternary thiospinels and their bifunctional electrocatalytic properties for oxygen reduction and evolution: The case of CuCo2S4 nanosheets. Inorg. Chem. Front. 2016, 3, 1501–1509. [Google Scholar] [CrossRef]
- Wei, W.; Mi, L.; Gao, Y.; Zheng, Z.; Chen, W.; Guan, X. Partial ion-exchange of nickel-sulfide-derived electrodes for high performance supercapacitors. Chem. Mater. 2014, 26, 3418–3426. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: An efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Zhao, C.; Kahol, P.K.; Kostoglou, N.; Mitterer, C.; Hinder, S.J.; Baker, M.A.; Constantinides, G.; Polychronopoulou, K.; et al. Electrodeposited nanostructured CoFe2O4 for overall water splitting and supercapacitor applications. Catalysts 2019, 9, 176. [Google Scholar] [CrossRef]
- Barhoum, A.; El-Maghrabi, H.H.; Iatsunskyi, I.; Coy, E.; Renard, A.; Salameh, C.; Matthieu, W.; Syreina, S.; Nada, A.A.; Stephanie, R.; et al. Atomic layer deposition of Pd nanoparticles on self-supported carbon-Ni/NiO-Pd nanofiber electrodes for electrochemical hydrogen and oxygen evolution reactions. J. Colloid Interface Sci. 2020, 569, 286–297. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Fast formation of single-unit-cell-thick and defect-rich layered double hydroxide nanosheets with highly enhanced oxygen evolution reaction for water splitting. Nano Res. 2018, 11, 1883–1894. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, M.; Wu, R. Strongly coupling of Co9S8/Zn-Co-S heterostructures rooted in carbon nanocages towards efficient oxygen evolution reaction. J. Catal. 2018, 361, 322–330. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Evans, D.; Yang, W. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale 2013, 5, 5312–5315. [Google Scholar] [CrossRef]
- McKendry, I.G.; Thenuwara, A.C.; Shumlas, S.L.; Peng, H.; Aulin, Y.V.; Chinnam, P.R.; Borguet, E.; Strongin, D.R.; Zdilla, M.J. Systematic Doping of Cobalt into Layered Manganese Oxide Sheets Substantially Enhances Water Oxidation Catalysis. Inorg. Chem. 2018, 57, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, J.; Yanzhang, R.; Du, M.; Wang, Q.; Gao, G.; Wu, J.; Wu, G.; Zhang, M.; Liu, B.; et al. When cubic cobalt sulfide meets layered molybdenum disulfide: A core-shell system toward synergetic electrocatalytic water splitting. Adv. Mater. 2015, 27, 4752–4759. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.J.; Kwon, K.; Cheon, J.Y.; Kleitz, F.; Joo, S.H. Ordered mesoporous Co3O4 spinels as stable, bifunctional, noble metal-free oxygen electrocatalysts. J. Mater. Chem. A 2013, 1, 9992–10001. [Google Scholar] [CrossRef]










| Electrocatalyst | Current Density (mA cm−2) | Overpotential (mV) | Tafel Slope (mV dec−1) | Reference |
|---|---|---|---|---|
| CuCo2S4 | 10 | 274 | 59 | This work |
| carbon-Ni/NiO-Pd | 10 | 380 | 72 | [40] |
| NiFe-LDH | 10 | 290 | 33.4 | [41] |
| Co9S8/Zn0.8Co0.2S@C | 10 | 292 | 52 | [42] |
| Co3O4/MnCo2O4 | 10 | 540 | N/A | [43] |
| K0.04 [Co0.42Mn0.58O2] | 10 | 420 | 110 | [44] |
| Co9S8@MoS2/CNFs | 10 | 430 | 61 | [45] |
| Mesoporous Co3O4 | 10 | 411 | 80 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Ma, P.; Yang, J.; Krishnan, S.; Kesavan, K.S.; Xing, R.; Liu, S. Facile Construction of Three-Dimensional Heterostructured CuCo2S4 Bifunctional Catalyst for Alkaline Water Electrolysis. Catalysts 2023, 13, 881. https://doi.org/10.3390/catal13050881
Li S, Ma P, Yang J, Krishnan S, Kesavan KS, Xing R, Liu S. Facile Construction of Three-Dimensional Heterostructured CuCo2S4 Bifunctional Catalyst for Alkaline Water Electrolysis. Catalysts. 2023; 13(5):881. https://doi.org/10.3390/catal13050881
Chicago/Turabian StyleLi, Shengnan, Pengli Ma, Jishuang Yang, Srinivasan Krishnan, Kannan S. Kesavan, Ruimin Xing, and Shanhu Liu. 2023. "Facile Construction of Three-Dimensional Heterostructured CuCo2S4 Bifunctional Catalyst for Alkaline Water Electrolysis" Catalysts 13, no. 5: 881. https://doi.org/10.3390/catal13050881
APA StyleLi, S., Ma, P., Yang, J., Krishnan, S., Kesavan, K. S., Xing, R., & Liu, S. (2023). Facile Construction of Three-Dimensional Heterostructured CuCo2S4 Bifunctional Catalyst for Alkaline Water Electrolysis. Catalysts, 13(5), 881. https://doi.org/10.3390/catal13050881