Long-Term Hydrogen Production from a Methanol–Water Solution Catalyzed by an Iridium Complex
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Methanol Concentration on the Efficiency of Hydrogen Production
2.2. Stability of the Catalyst at High Temperature for a Long Period of Time
2.3. Optimization of Reaction Conditions for Long-Term Hydrogen Production
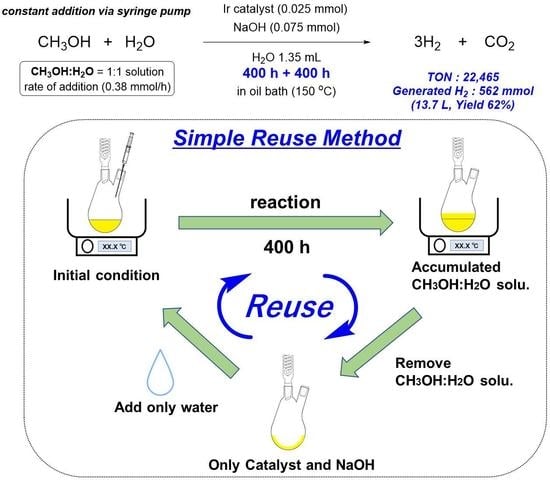
2.4. Procedure for the Reactivation and Reuse of the Catalyst
3. Materials and Methods
3.1. General
3.2. Dehydrogenation from Methanol and Water (Measured by the Gas Burette)
3.3. Procedure for the Stability Test of the Catalyst 2 in Water
3.4. Procedure for the Stability Test of the Catalyst 2 in 0.067 M NaOH Aq
3.5. Dehydrogenation from Methanol and Water (Measured by the Milli-Gas Counter)
3.6. Procedure for Long-Term Continuous Hydrogen Production by the Reuse Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rostrup-Nielsen, J.R. Production of Synthesis Gas. Catal. Today 1993, 18, 305–324. [Google Scholar] [CrossRef]
- Besenbacher, F.; Chorkendorff, I.; Clausen, B.S.; Hammer, B.; Molenbroek, A.M.; Nørskov, J.K.; Stensgaard, I. Design of a Surface Alloy Catalyst for Steam Reforming. Science 1998, 279, 1913–1915. [Google Scholar] [CrossRef]
- Trimm, D.L. Catalysts for the Control of Coking during Steam Reforming. Catal. Today 1999, 49, 3–10. [Google Scholar] [CrossRef]
- Osaki, T.; Mori, T. Role of Potassium in Carbon-Free CO2 Reforming of Methane on K-Promoted Ni/Al2O3 Catalysts. J. Catal. 2001, 204, 89–97. [Google Scholar] [CrossRef]
- Shimura, K.; Kato, S.; Yoshida, T.; Itoh, H.; Hattori, T.; Yoshida, H. Photocatalytic Steam Reforming of Methane over Sodium Tantalate. J. Phys. Chem. C 2010, 114, 3493–3503. [Google Scholar] [CrossRef]
- Duarte, R.B.; Krumeich, F.; Van Bokhoven, J.A. Structure, Activity, and Stability of Atomically Dispersed Rh in Methane Steam Reforming. ACS Catal. 2014, 4, 1279–1286. [Google Scholar] [CrossRef]
- Mondal, T.; Pant, K.K.; Dalai, A.K. Mechanistic Kinetic Modeling of Oxidative Steam Reforming of Bioethanol for Hydrogen Production over Rh−Ni/CeO2−ZrO2 Catalyst. Ind. Eng. Chem. Res. 2016, 55, 86–98. [Google Scholar] [CrossRef]
- Che, F.; Gray, J.T.; Ha, S.; McEwen, J.S. Improving Ni Catalysts Using Electric Fields: A DFT and Experimental Study of the Methane Steam Reforming Reaction. ACS Catal. 2017, 7, 551–562. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, T. Manufacture of Hydrogen. Catal. Today 2005, 106, 293–296. [Google Scholar] [CrossRef]
- Murcia-Mascarós, S.; Navarro, R.M.; Gómez-Sainero, L.; Costantino, U.; Nocchetti, M.; Fierro, J.L.G. Regular Article. J. Catal. 2001, 2, 338–347. [Google Scholar] [CrossRef]
- Reitz, T.L.; Lee, P.L.; Lang, J.C.; Lang, J.C.; Kung, H.H. Time-Resolved XANES Investigation of CuO/ZnO in the Oxidative Methanol Reforming Reaction. J. Catal. 2001, 199, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.S.; Chung, S.C. Kinetics of Hydrogen Production of Methanol Reformation Using Cu/ZnO/Al2O3 Catalyst. J. Comb. Chem. 2007, 9, 990–997. [Google Scholar] [CrossRef]
- Halevi, B.; Lin, S.; Roy, A.; Zhang, H.; Jeroro, E.; Vohs, J.; Wang, Y.; Guo, H.; Datye, A.K. High CO2 Selectivity of ZnO Powder Catalysts for Methanol Steam Reforming. J. Phys. Chem. C 2013, 117, 6493–6503. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Remiro, A.; Epron, F.; Bion, N.; Aguayo, A.T.; Bilbao, J.; Gayubo, A.G. Comparison of Noble Metal- and Copper-Based Catalysts for the Step of Methanol Steam Reforming in the Dimethyl Ether Steam Reforming Process. Ind. Eng. Chem. Res. 2016, 55, 3546–3555. [Google Scholar] [CrossRef]
- Mayr, L.; Köpfle, N.; Klötzer, B.; Götsch, T.; Bernardi, J.; Schwarz, S.; Keilhauer, T.; Armbrüster, M.; Penner, S. Microstructural and Chemical Evolution and Analysis of a Self-Activating CO2-Selective Cu-Zr Bimetallic Methanol Steam Reforming Catalyst. J. Phys. Chem. C 2016, 120, 25395–25404. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; et al. Low-Temperature Hydrogen Production from Water and Methanol Using Pt/α-MoC Catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Ibrahim, J.J.; Fu, Y.; Kong, W.; Zhang, J.; Sun, Y. Low-Temperature Hydrogen Production from Methanol Steam Reforming on Zn-Modified Pt/MoC Catalysts. Appl. Catal. B 2019, 264, 118500. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, Z.-Q. Hydrogen Generation from Methanol Reforming for Fuel Cell Applications: A Review. J. Cent. South Univ. 2020, 27, 1074–1103. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Q.; Peng, M.; Li, A.; Yao, S.; Tian, S.; Liu, X.; Li, A.; Jiang, Z.; Gao, R.; et al. Atomically Dispersed Ni/α-MoC Catalyst for Hydrogen Production from Methanol/Water. J. Am. Chem. Soc. 2021, 143, 309–317. [Google Scholar] [CrossRef]
- Nielsen, M.; Alberico, E.; Baumann, W.; Drexler, H.J.; Junge, H.; Gladiali, S.; Beller, M. Low-Temperature Aqueous-Phase Methanol Dehydrogenation to Hydrogen and Carbon Dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef]
- Boucher, M.B.; Marcinkowski, M.D.; Liriano, M.L.; Murphy, C.J.; Lewis, E.A.; Jewell, A.D.; Mattera, M.F.G.; Kyriakou, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Molecular-Scale Perspective of Water-Catalyzed Methanol Dehydrogenation to Formaldehyde. ACS Nano 2013, 7, 6181–6187. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lugo, R.E.; Trincado, M.; Vogt, M.; Tewes, F.; Santiso-Quinones, G.; Grützmacher, H. A Homogeneous Transition Metal Complex for Clean Hydrogen Production from Methanol–Water Mixtures. Nat. Chem. 2013, 5, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Bielinski, E.A.; Förster, M.; Zhang, Y.; Bernskoetter, W.H.; Hazari, N.; Holthausen, M.C. Base-Free Methanol Dehydrogenation Using a Pincer-Supported Iron Compound and Lewis Acid Co-Catalyst. ACS Catal. 2015, 5, 2404–2415. [Google Scholar] [CrossRef]
- Campos, J.; Sharninghausen, L.S.; Manas, M.G.; Crabtree, R.H. Methanol Dehydrogenation by Iridium N-Heterocyclic Carbene Complexes. Inorg. Chem. 2015, 54, 5079–5084. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.N.; Yuan, M.; Comin, R.; Voznyy, O.; Beauregard, E.M.; Hoogland, S.; Buin, A.; Kirmani, A.R.; Zhao, K.; Amassian, A.; et al. Ligand-Stabilized Reduced-Dimensionality Perovskites. J. Am. Chem. Soc. 2016, 138, 2649–2655. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Lan, J.; Liang, R.; Xia, Y.; Qin, L.; Chung, L.W.; Zheng, Z. New Tricks for an Old Dog: Grubbs Catalysts Enable Efficient Hydrogen Production from Aqueous-Phase Methanol Reforming. ACS Catal. 2022, 12, 2212–2222. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, Y.; Ma, C.; Wang, Q.; Qin, L.; Zhu, X.; Zheng, Z. Hydrogen Production via the Aqueous-Phase Reforming of Methanol Catalyzed by Ru(II) Complexes of PNNP Ligands. Inorg. Chem. Front. 2023, 10, 756–767. [Google Scholar] [CrossRef]
- Alberico, E.; Sponholz, P.; Cordes, C.; Nielsen, M.; Drexler, H.J.; Baumann, W.; Junge, H.; Beller, M. Selective Hydrogen Production from Methanol with a Defined Iron Pincer Catalyst under Mild Conditions. Angew. Chem. Int. Ed. 2013, 52, 14162–14166. [Google Scholar] [CrossRef]
- Hu, P.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Reusable Homogeneous Catalytic System for Hydrogen Production from Methanol and Water. ACS Catal. 2014, 4, 2649–2652. [Google Scholar] [CrossRef]
- Prichatz, C.; Alberico, E.; Baumann, W.; Junge, H.; Beller, M. Iridium–PNP Pincer Complexes for Methanol Dehydrogenation at Low Base Concentration. ChemCatChem 2017, 9, 1891–1896. [Google Scholar] [CrossRef]
- Andérez-Fernández, M.; Vogt, L.K.; Fischer, S.; Zhou, W.; Jiao, H.; Garbe, M.; Elangovan, S.; Junge, K.; Junge, H.; Ludwig, R.; et al. A Stable Manganese Pincer Catalyst for the Selective Dehydrogenation of Methanol. Angew. Chem. Int. Ed. 2017, 56, 559–562. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Kar, S.; Rauch, M.; Montag, M.; Ben-David, Y.; Milstein, D. Efficient Base-Free Aqueous Reforming of Methanol Homogeneously Catalyzed by Ruthenium Exhibiting a Remarkable Acceleration by Added Catalytic Thiol. J. Am. Chem. Soc. 2021, 143, 17284–17291. [Google Scholar] [CrossRef]
- Fujita, K.; Tanino, N.; Yamaguchi, R. Ligand-Promoted Dehydrogenation of Alcohols Catalyzed by Cp*Ir Complexes. A New Catalytic System for Oxidant-Free Oxidation of Alcohols. Org. Lett. 2007, 9, 109–111. [Google Scholar] [CrossRef]
- Fujita, K.; Yoshida, T.; Imori, Y.; Yamaguchi, R. Dehydrogenative Oxidation of Primary and Secondary Alcohols Catalyzed by a Cp*Ir Complex Having a Functional C,N-Chelate Ligand. Org. Lett. 2011, 13, 2278–2281. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Cooperative Catalysis by Iridium Complexes with a Bipyridonate Ligand: Versatile Dehydrogenative Oxidation of Alcohols and Reversible Dehydrogenation–Hydrogenation between 2-Propanol and Acetone. Angew. Chem. Int. Ed. 2012, 51, 12790–12794. [Google Scholar] [CrossRef]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Dehydrogenative Oxidation of Alcohols in Aqueous Media Using Water-Soluble and Reusable Cp*Ir Catalysts Bearing a Functional Bipyridine Ligand. J. Am. Chem. Soc. 2012, 134, 3643–3646. [Google Scholar] [CrossRef]
- Zeng, G.; Sakaki, S.; Fujita, K.; Sano, H.; Yamaguchi, R. Efficient Catalyst for Acceptorless Alcohol Dehydrogenation: Interplay of Theoretical and Experimental Studies. ACS Catal. 2014, 4, 1010–1020. [Google Scholar] [CrossRef]
- Fujita, K.; Inoue, T.; Tanaka, T.; Jeong, J.; Furukawa, S.; Yamaguchi, R. Iridium Complex Catalyzed Hydrogen Production from Glucose and Various Monosaccharides. Catalysts 2021, 11, 891. [Google Scholar] [CrossRef]
- Toyooka, G.; Tanaka, T.; Kitayama, K.; Kobayashi, N.; Watanabe, T.; Fujita, K. Hydrogen Production from Cellulose Catalyzed by an Iridium Complex in Ionic Liquid under Mild Conditions. Catal. Sci. Technol. 2021, 11, 2273–2279. [Google Scholar] [CrossRef]
- Fujita, K.; Kawahara, R.; Aikawa, T.; Yamaguchi, R. Hydrogen Production from a Methanol–Water Solution Catalyzed by an Anionic Iridium Complex Bearing a Functional Bipyridonate Ligand under Weakly Basic Conditions. Angew. Chem. Int. Ed. 2015, 54, 9057–9060. [Google Scholar] [CrossRef] [PubMed]







 | |||||
| Entry | X | Y | Ratio | Generated H2 | TON |
| [mmol] | [mmol] | [X:Y] | [mL] | ||
| 1 | 25 | 125 | 1:5 | 1358 | 556 |
| 2 | 75 | 75 | 1:1 | 278 | 116 |
| 3 | 125 | 25 | 5:1 | 240 | 99 |
 | ||||||
| Entry | Cat. | NaOH | X | Y | Time | TON |
|---|---|---|---|---|---|---|
| [mmol] | [mmol] | [mL] | [mmol/h] | [h] | ||
| 1 | 0.1 | 0.3 | 2.7 | 1.51 | 350 | 11,104 |
| 2 | 0.05 | 0.15 | 1.35 | 0.761 | 450 | 14,951 |
| 3 | 0.025 | 0.075 | 0.68 | 0.379 | - | - |
| 4 | 0.025 | 0.075 | 1.35 | 0.379 | >500 | >15,001 |
| 5 | 0.025 | 0.075 | 2.0 | 0.379 | 100 | 3228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, S.; Kubota, K.; Wang, H.; Gong, H.; Kajita, S.; Fujita, K.-i. Long-Term Hydrogen Production from a Methanol–Water Solution Catalyzed by an Iridium Complex. Catalysts 2023, 13, 1027. https://doi.org/10.3390/catal13061027
Furukawa S, Kubota K, Wang H, Gong H, Kajita S, Fujita K-i. Long-Term Hydrogen Production from a Methanol–Water Solution Catalyzed by an Iridium Complex. Catalysts. 2023; 13(6):1027. https://doi.org/10.3390/catal13061027
Chicago/Turabian StyleFurukawa, Shohichi, Kaito Kubota, Han Wang, Haotong Gong, Shumpei Kajita, and Ken-ichi Fujita. 2023. "Long-Term Hydrogen Production from a Methanol–Water Solution Catalyzed by an Iridium Complex" Catalysts 13, no. 6: 1027. https://doi.org/10.3390/catal13061027
APA StyleFurukawa, S., Kubota, K., Wang, H., Gong, H., Kajita, S., & Fujita, K. -i. (2023). Long-Term Hydrogen Production from a Methanol–Water Solution Catalyzed by an Iridium Complex. Catalysts, 13(6), 1027. https://doi.org/10.3390/catal13061027