Vanadium Complexes Derived from O,N,O-tridentate 6-bis(o-hydroxyalkyl/aryl)pyridines: Structural Studies and Use in the Ring-Opening Polymerization of ε-Caprolactone and Ethylene Polymerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of V Complexes
2.1.1. Use of 2,6-bis(o-hydroxyaryl)pyridine, 2,6-{HOC(Ph)2CH2}2(NC5H3), LH2
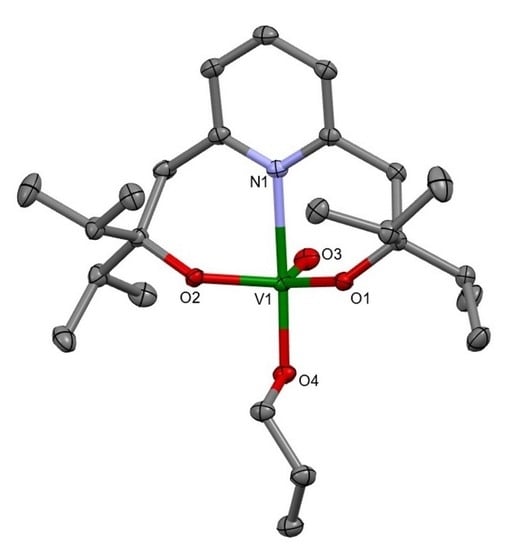
2.1.2. Use of 2,6-bis(o-hydroxy-i-propyl)pyridine L1H2
2.1.3. Use of the Bis(Methylpyridine)-Substituted Alcohol (tBu)C(OH)[CH2(C5H3Me-5)]2
2.2. Ring Opening Polymerization (ROP) Studies
2.2.1. ROP of ε-Caprolactone (ε-CL)
2.2.2. ROP of δ-Valerolactone (δ-VL)
2.2.3. Ring Opening Polymerization Studies of Rac-Lactide (r-LA)
2.3. Ethylene Polymerization Studies
3. Materials and Methods
3.1. General
3.2. Preparation of [VO(OiPr)L] (1)
3.3. Preparation of [V({OC(Ph)2CH2}2(cis-NC5H3))2] (2)
3.4. Preparation of [V({OC(Ph)2CH2}2(trans-NC5H3))2] (3∙2THF)
3.5. Preparation of [VO(OnPr)L1] (4)
3.6. Preparation of [VO(OiPr)L1] (5)
3.7. Preparation of [VO(μ-O)(L2)]2 (6)
3.8. Procedure for ROP of ε-Caprolactone
3.9. Kinetic Studies
3.10. Procedure for Ethylene Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czigany, T.; Ronkay, F. The coronavirus and plastics. Express Polym. Lett. 2020, 14, 510–511. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Reichman, J.H. Intellectual Property in the Twenty-First Century: Will the Developing Countries Lead or Follow? In Intellectual Property Rights: Legal and Economic Challenges for Development; Cimoli, M., Dosi, G., Maskus, K.E., Okediji, R.L., Reichman, J.H., Eds.; Oxford University Press: Oxford, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.L.P.; Prata, J.C.; Duarte, A.C.; Barcelò, D.; Rocha-Santos, T. An urgent call to think globally and act locally on landfill disposable plastics under and after COVID-19 pandemic: Pollution prevention and technological (Bio) remediation solutions. Chem. Eng. J. 2021, 426, 131201. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable Synthetic Polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Kim, Y.K. The use of polyolefins in industrial and medical applications. In The Textile Institute Book Series, Polyolefin Fibres, 2nd ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 135–155. ISBN 9780081011324. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C. Metal Catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Zhang, X.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an Enabling Science for Sustainable Polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef]
- Chen, C. Designing catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Rev. Chem. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Dove, A.P. Organic Catalysis for Ring-Opening Polymerization. ACS Macro Lett. 2012, 1, 1409–1412. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef]
- Williams, C.K.; Hillmyer, M.A. Polymers from Renewable Resources: A Perspective for a Special Issue of Polymer Reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic Polymer Scaffolds for Tissue Engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of Polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Lecomte, P.; Jér, C. Synthetic Biodegradable Polymers; Rieger, B., Künkel, A., Coates, G., Reichardt, R., Dinjus, E., Zevaco, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 245, pp. 173–211. ISBN 978-3642271533. [Google Scholar]
- Redshaw, C. Metallocalixarene catalysts: α-olefin polymerization and ROP of cyclic esters. Dalton Trans. 2016, 45, 9018–9030. [Google Scholar] [CrossRef]
- Redshaw, C. Use of Metal Catalysts Bearing Schiff Base Macrocycles for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2017, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Redshaw, C.; Warford, L.; Dale, S.H.; Elsegood, M.R.J. Vanadyl complexes bearing bi- and triphenolate chelate ligands: Highly active ethylene polymerisation procatalysts. Chem. Commun. 2004, 1954–1955. [Google Scholar] [CrossRef]
- Redshaw, C.; Rowan, M.A.; Homden, D.M.; Dale, S.H.; Elsegood, M.R.J.; Matsui, S.; Matsuura, S. Vanadyl C and N-capped tris(phenolate) complexes: Influence of pro-catalyst geometry on catalytic activity. Chem. Commun. 2006, 3329–3331. [Google Scholar] [CrossRef]
- Redshaw, C.; Rowan, M.A.; Warford, L.; Homden, D.M.; Arbaoui, A.; Elsegood, M.R.J.; Dale, S.H.; Yamato, T.; Casas, C.P.; Matsui, S.; et al. Oxo- and Imidovanadium Complexes Incorporating Methylene- and Dimethyleneoxa-Bridged Calix[3]- and -[4]arenes: Synthesis, Structures and Ethylene Polymerisation Catalysis. Chem.-Eur. J. 2007, 13, 1090–1107. [Google Scholar] [CrossRef]
- Homden, D.; Redshaw, C.; Warford, L.; Hughes, D.L.; Wright, J.A.; Dale, S.H.; Elsegood, M.R.J. Synthesis, structure and ethylene polymerisation behaviour of vanadium(iv and v) complexes bearing chelating aryloxides. Dalton Trans. 2009, 8900–8910. [Google Scholar] [CrossRef]
- Lorber, C.; Despagnet-Ayoub, E.; Vendier, L.; Arbaoui, A.; Redshaw, C. Amine influence in vanadium-based ethylene polymerisation pro-catalysts bearing bis(phenolate) ligands with ‘pendant’ arms. Catal. Sci. Technol. 2011, 1, 489–494. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of Vanadium Complex Catalysts for Precise Olefin Polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Q.; Li, Y.-S. Well-defined vanadium complexes as the catalysts for olefin polymerization. Coord. Chem. Rev. 2011, 255, 2303–2314. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, K.-Q.; Walton, M.J.; Wright, J.A.; Frese, J.W.A.; Elsegood, M.R.J.; Xing, Q.; Sun, W.-H.; Redshaw, C. Vanadyl complexes bearing bi-dentate phenoxyimine ligands: Synthesis, structural studies and ethylene polymerization capability. Dalton Trans. 2014, 43, 8300–8310. [Google Scholar] [CrossRef]
- Phillips, A.M.F.; Suo, H.; Silva, M.D.F.C.G.D.; Pombeiro, A.J.; Sun, W.-H. Recent developments in vanadium-catalyzed olefin coordination polymerization. Coord. Chem. Rev. 2020, 416, 213332. [Google Scholar] [CrossRef]
- Qian, J.; Comito, R.J. A Robust Vanadium(V) Tris(2-pyridyl)borate Catalyst for Long-Lived High-Temperature Ethylene Polymerization. Organometallics 2021, 40, 1817–1821. [Google Scholar] [CrossRef]
- Wu, R.; Niu, Z.; Huang, L.; Xia, Z.; Feng, Z.; Qi, Y.; Dai, Q.; Cui, L.; He, J.; Bai, C. Vanadium complexes bearing the bulky bis(imino)pyridine ligands: Good thermal stability toward ethylene polymerization. Eur. Polym. J. 2022, 180, 111569. [Google Scholar] [CrossRef]
- Suo, H.; Phillips, A.M.F.; Satrudhar, M.; Martins, L.M.D.R.S.; Silva, M.d.F.G.d.; Pombeiro, A.J.L.; Han, M.; Sun, W. Achieving ultra-high molecular weight polyethylenes by vanadium aroylhydrazine-arylolates. J. Polym. Sci. 2023, 61, 482–490. [Google Scholar] [CrossRef]
- Qian, J.; Comito, R.J. Ethylene Polymerization with Thermally Robust Vanadium(III) Tris(2-pyridyl)borate Complexes. Organometallics 2023. [Google Scholar] [CrossRef]
- Homden, D.M.; Redshaw, C.; Hughes, D.L. Vanadium Complexes Possessing N2O2S2-Based Ligands: Highly Active Procatalysts for the Homopolymerization of Ethylene and Copolymerization of Ethylene/1-hexene. Inorg. Chem. 2007, 46, 10827–10839. [Google Scholar] [CrossRef]
- Redshaw, C.; Walton, M.; Michiue, K.; Chao, Y.; Walton, A.; Elo, P.; Sumerin, V.; Jiang, C.; Elsegood, M.R.J. Vanadyl calix[6]arene complexes: Synthesis, structural studies and ethylene homo-(co-)polymerization capability. Dalton Trans. 2015, 44, 12292–12303. [Google Scholar] [CrossRef]
- Redshaw, C.; Walton, M.J.; Elsegood, M.R.J.; Prior, T.J.; Michiue, K. Vanadium(v) tetra-phenolate complexes: Synthesis, structural studies and ethylene homo-(co-)polymerization capability. RSC Adv. 2015, 5, 89783–89796. [Google Scholar] [CrossRef] [Green Version]
- Redshaw, C.; Walton, M.J.; Lee, D.S.; Jiang, C.; Elsegood, M.R.J.; Michiue, K. Vanadium(V) Oxo and Imido Calix[8]arene Complexes: Synthesis, Structural Studies, and Ethylene Homo/Copolymerisation Capability. Chem.-Eur. J. 2015, 21, 5199–5210. [Google Scholar] [CrossRef] [Green Version]
- Diteepeng, N.; Tang, X.; Hou, X.; Li, Y.-S.; Phomphrai, K.; Nomura, K. Ethylene polymerisation and ethylene/norbornene copolymerisation by using aryloxo-modified vanadium(v) complexes containing 2,6-difluoro-, dichloro-phenylimido complexes. Dalton Trans. 2015, 44, 12273–12281. [Google Scholar] [CrossRef]
- Zanchin, G.; Bertini, F.; Vendier, L.; Ricci, G.; Lorber, C.; Leone, G. Copolymerization of ethylene with propylene and higher α-olefins catalyzed by (imido)vanadium(iv) dichloride complexes. Polym. Chem. 2019, 10, 6200–6216. [Google Scholar] [CrossRef]
- Ochędzan-Siodłak, W.; Siodłak, D.; Banaś, K.; Halikowska, K.; Wierzba, S.; Doležal, K. Naturally Occurring Oxazole Structural Units as Ligands of Vanadium Catalysts for Ethylene-Norbornene (Co)polymerization. Catalysts 2021, 11, 923. [Google Scholar] [CrossRef]
- Wu, R.; Niu, Z.; Huang, L.; Yang, Y.; Xia, Z.; Fan, W.; Dai, Q.; Cui, L.; He, J.; Bai, C. Thermally stable vanadium complexes supported by the iminophenyl oxazolinylphenylamine ligands: Synthesis, characterization and application for ethylene (co-)polymerization. Dalton Trans. 2021, 50, 16067–16075. [Google Scholar] [CrossRef]
- Białek, M.; Fryga, J.; Spaleniak, G.; Matsko, M.A.; Hajdasz, N. Ethylene homo- and copolymerization catalyzed by vanadium, zirconium, and titanium complexes having potentially tridentate Schiff base ligands. J. Catal. 2021, 400, 184–194. [Google Scholar] [CrossRef]
- Nie, J.; Ren, F.; Li, Z.; Tian, K.; Zou, H.; Hou, X. Design and synthesis of binuclear vanadium catalysts for copolymerization of ethylene and polar monomers. Polym. Chem. 2022, 13, 3876–3881. [Google Scholar] [CrossRef]
- Ge, F.; Dan, Y.; Al-Khafaji, Y.; Prior, T.J.; Jiang, L.; Elsegood, M.R.J.; Redshaw, C. Vanadium(v) phenolate complexes for ring opening homo- and co-polymerisation of ε-caprolactone, l-lactide and rac-lactide. RSC Adv. 2016, 6, 4792–4802. [Google Scholar] [CrossRef] [Green Version]
- Białek, M.; Fryga, J.; Spaleniak, G.; Dziuk, B. Ring opening polymerization of ε-caprolactone initiated by titanium and vanadium complexes of ONO-type schiff base ligand. J. Polym. Res. 2021, 28, 79. [Google Scholar] [CrossRef]
- Arbaoui, A.; Redshaw, C.; Homden, D.M.; Wright, J.A.; Elsegood, M.R.J. Vanadium-based imido-alkoxide pro-catalysts bearing bisphenolate ligands for ethylene and ε-caprolactone polymerisation. Dalton Trans. 2009, 8911–8922. [Google Scholar] [CrossRef] [PubMed]
- Clowes, L.; Redshaw, C.; Hughes, D.L. Vanadium-Based Pro-Catalysts Bearing Depleted 1,3-Calix[4]arenes for Ethylene or ε-Caprolactone Polymerization. Inorg. Chem. 2011, 50, 7838–7845. [Google Scholar] [CrossRef] [PubMed]
- Clowes, L.; Walton, M.; Redshaw, C.; Chao, Y.; Walton, A.; Elo, P.; Sumerin, V.; Hughes, D.L. Vanadium(iii) phenoxyimine complexes for ethylene or ε-caprolactone polymerization: Mononuclear versus binuclear pre-catalysts. Catal. Sci. Technol. 2013, 3, 152–160. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, K.-Q.; Walton, M.; Wright, J.A.; Hughes, D.L.; Elsegood, M.R.J.; Michiue, K.; Sun, X.; Redshaw, C. Tri- and tetra-dentate imine vanadyl complexes: Synthesis, structure and ethylene polymerization/ring opening polymerization capability. Dalton Trans. 2014, 43, 16698–16706. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Prior, T.J.; Elsegood, M.R.J.; Semikolenova, N.V.; Soshnikov, I.E.; Bryliakov, K.P.; Chen, K.; Redshaw, C. Vanadium complexes derived from oxacalix[6]arenes: Structural studies and use in the ring opening homo-/co-polymerization of ε-caprolactone/δ-valerolactone and ethylene polymerization. Catal. Sci. Technol. 2021, 11, 624–636. [Google Scholar] [CrossRef]
- Ishikura, H.; Neven, R.; Lange, T.; Galetová, A.; Blom, B.; Romano, D. Developments in vanadium-catalysed polymerisation reactions: A review. Inorg. Chim. Acta 2021, 515, 120047. [Google Scholar] [CrossRef]
- Cusi, K.; Cukier, S.; DeFronzo, R.A.; Torres, M.; Puchulu, F.M.; Redondo, J.C.P. Vanadyl Sulfate Improves Hepatic and Muscle Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 1410–1417. [Google Scholar] [CrossRef]
- Redshaw, C.; Elsegood, M.R.J.; Wright, J.A.; Baillie-Johnson, H.; Yamato, T.; De Giovanni, S.; Mueller, A. Cellular uptake of a fluorescent vanadyl sulfonylcalix[4]arene. Chem. Commun. 2012, 48, 1129–1131. [Google Scholar] [CrossRef]
- Suzuki, N.; Kobayashi, G.; Hasegawa, T.; Masuyama, Y. Syntheses and structures of titanium complexes having O,N,O-tridentate ligands and their catalytic ability for ethylene polymerization. J. Organomet. Chem. 2012, 717, 23–28. [Google Scholar] [CrossRef]
- Lai, F.-J.; Huang, T.-W.; Chang, Y.-L.; Chang, H.-Y.; Lu, W.-Y.; Ding, S.; Chen, H.-Y.; Chiu, C.-C.; Wu, K.-H. Titanium complexes bearing 2,6-Bis(o-hydroxyalkyl)pyridine ligands in the ring-opening polymerization of L-Lactide and ε-caprolactone. Polymer 2020, 204, 122860. [Google Scholar] [CrossRef]
- Nakayama, Y.; Ikushima, N.; Nakamura, A. Cis-Specific Polymerization of Norbornene Catalyzed by Tungsten Based Complex Catalysts Bearing an O–N–O Tridentate Ligand. Chem. Lett. 1997, 26, 861–862. [Google Scholar] [CrossRef]
- Evans, W.J.; Anwander, R.; Berlekamp, U.H.; Ziller, J.W. Synthesis and Structure of Lanthanide Complexes Derived from the O,N-Chelating, Bis(methylpyridine)-Substituted Alcohol HOC(CMe3)(2-CH2NC5H3Me-6)2. Inorg. Chem. 1995, 34, 3583–3588. [Google Scholar] [CrossRef]
- Gibson, V.C.; Newton, C.; Redshaw, C.; Solan, G.A.; White, A.J.P.; Williams, D.J. Low valent chromium complexes bearing N,O-chelating pyridyl-enolate ligands [OC(But)(=2-CHN5H3Me-x)]− (x = 3–6). Dalton Trans. 2003, 4612–4617. [Google Scholar] [CrossRef]
- Berg, J.M.; Holm, R.H. Model for the active site of oxo-transfer molybdoenzymes: Synthesis, structure, and properties. J. Am. Chem. Soc. 1985, 107, 917–925. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Bai, F.; Yue, J.; Woo, H.-G. Living Ring-Opening Polymerization of l-Lactide Catalyzed by Red-Al. Organometallics 2004, 23, 1411–1415. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Shubin, A.A.; Bryliakov, K.P.; Zakharov, V.A.; Redshaw, C.; Talsi, E.P. EPR Monitoring of Vanadium(IV) Species Formed upon Activation of Vanadium(V) Polyphenolate Precatalysts with AlR2Cl and AlR2Cl/Ethyltrichloroacetate (R = Me, Et). Organometallics 2009, 28, 6714–6720. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Shubin, A.A.; Zakharov, V.A.; Redshaw, C.; Talsi, E.P. An EPR Study of the V(IV) Species Formed Upon Activation of a Vanadyl Phenoxyimine Polymerization Catalyst with AlR3and AlR2Cl (R = Me, Et). Macromol. Chem. Phys. 2009, 210, 542–548. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.; Zakharov, V.A.; Redshaw, C.; Talsi, E.P. An EPR study of the vanadium species formed upon interaction of vanadyl N and C-capped tris(phenolate) complexes with AlEt3 and AlEt2Cl. J. Mol. Catal. A Chem. 2009, 303, 23–29. [Google Scholar] [CrossRef]
- Deng, S.; Liu, Z.; Liu, B.; Jin, Y. Unravelling the Role of Al-alkyl Cocatalyst for the VOx/SiO2 Ethylene Polymerization Catalyst: Diethylaluminum Chloride vs. Triethylaluminum. ChemCatChem 2021, 13, 2278–2292. [Google Scholar] [CrossRef]
- Manzer, L. Tetrahydrofuran Complexes of Selected Early Transition-Metals. In Inorganic Syntheses; Fackler, J.P., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1982; Volume 21, pp. 135–140. ISBN 978-0-470-13287-6. [Google Scholar]
- Edema, J.J.H.; Stauthamer, W.; Van Bolhuis, F.; Gambarotta, S.; Smeets, W.J.J.; Spek, A.L. Novel vanadium(II) amine complexes: A facile entry in the chemistry of divalent vanadium. Synthesis and characterization of mononuclear L4VCl2 [L = amine, pyridine]: X-ray structures of trans-(TMEDA)2VCl2 [TMEDA = N,N,N′,N′-tetramethylethylenediamine] and trans-Mz2V(py)2 [Mz = o-C6H4CH2N(CH3)2, py = pyridine]. Inorg. Chem. 1990, 29, 1302–1306. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]







| Entry | Cat. | (CL):(Cat) | T/°C | Conv a (%) | Mn b | Mn,Cal c | D d |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 500:1 | 15 | 0 | - | - | - |
| 2 | 1 | 500:1 | 60 | 20 | 270 | 11,430 | 1.63 |
| 3 | 1 | 500:1 | 90 | 23 | 380 | 26,270 | 2.10 |
| 4 | 1 | 500:1 | 130 | >99 | 6760 | 56,520 | 2.00 |
| 5 e | 1 | 500:1 | 130 | >99 | 7610 | 56,520 | 1.67 |
| 6 | 1 | 100:1 | 130 | 50 | 550 | 5720 | 1.67 |
| 7 | 1 | 1000:1 | 130 | 7 | 250 | 8010 | 1.31 |
| 8 | 1 | 250:1 | 130 | 47 | 880 | 13,430 | 1.81 |
| 9 | 2 | 500:1 | 130 | 22 | 440 | 12,560 | 2.86 |
| 10 | 3 | 500:1 | 130 | 19 | - | - | - |
| 11 | 4 | 500:1 | 70 | 2 | 1275 | 1160 | 2.23 |
| 12 | 4 | 500:1 | 130 | 92 | 2510 | 52,520 | 1.35 |
| 13 | 4 | 100:1 | 130 | 64 | 270 | 7320 | 1.55 |
| 14 | 5 | 500:1 | 70 | 58 | 500 | 33,120 | 1.45 |
| 15 | 5 | 100:1 | 130 | 98 | 440 | 11,200 | 2.42 |
| 16 e | 5 | 500:1 | 130 | >99 | 4410 | 56,520 | 1.59 |
| 17 | 6 | 500:1 | 130 | 63 | 28,890/2260 | 35,970 | 1.22/1.17 |
| 18 e | 6 | 500:1 | 90 | 49 | 620 | 27,980 | 1.62 |
| 19 | I | 500:1 | 130 | >99 | 13,770 | 56,520 | 1.67 |
| 20 e | I | 500:1 | 130 | >99 | 2070 | 56,520 | 1.18 |
| Entry | Cat. | (CL):(Cat) | Conv a (%) | Mn b | Mn,Cal c | D d |
|---|---|---|---|---|---|---|
| 1 | 1 | 500:1 | 81 | 5280 | 46,240 | 2.14 |
| 2 e | 1 | 500:1 | 85 | 3090 | 48,530 | 2.06 |
| 3 | 2 | 500:1 | 83 | 2990 | 47,390 | 1.24 |
| 4 | 3 | 500:1 | 84 | 2350 | 47,960 | 1.43 |
| 5 | 4 | 500:1 | 86 | 2460 | 49,100 | 2.02 |
| 6 e | 4 | 500:1 | 98 | 2080 | 55,950 | 2.92 |
| 7 | 5 | 500:1 | >99 | 5960 | 56,520 | 1.17 |
| 8 e | 5 | 500:1 | >99 | 3460 | 56,520 | 1.09 |
| 9 | 6 | 500:1 | >99 | 2270/200 | 56,520 | 1.47 |
| 10 e | 6 | 500:1 | 49 | 3580/280 | 27,980 | 1.04 |
| 11 | I | 500:1 | 74 | 6100 | 42,250 | 2.26 |
| 12 e | I | 500:1 | >99 | 8720 | 56,520 | 1.94 |
| Entry | Cat. | [VL]:[Cat] | Conv a (%) | Mn b | Mn,Cal c | D d |
|---|---|---|---|---|---|---|
| 1 | 1 | 500:1 | 87 | 3100 | 43,570 | 1.35 |
| 2 e | 1 | 500:1 | 91 | 920 | 45,570 | 2.00 |
| 3 | 2 | 500:1 | 77 | 500 | 38,580 | 1.49 |
| 4 e | 2 | 500:1 | 83 | 330 | 41,570 | 1.27 |
| 5 | 3 | 500:1 | 75 | 500 | 49,580 | 1.55 |
| 6 | 4 | 500:1 | 97 | 3420 | 48,580 | 1.46 |
| 7 e | 4 | 500:1 | 99 | 1550 | 49,580 | 1.87 |
| 8 | 5 | 500:1 | 89 | 2900 | 44,570 | 1.49 |
| 9 e | 5 | 500:1 | 90 | 3990 | 45,070 | 1.18 |
| 10 | 6 | 500:1 | 75 | 380 | 37,560 | 1.60 |
| 11 e | 6 | 500:1 | 54 | 390 | 27,050 | 2.16 |
| 12 | I | 500:1 | 72 | 500 | 36,060 | 1.76 |
| 13 e | I | 500:1 | 46 | 360 | 23,050 | 1.85 |
| Entry | Cat. | (rLA):(Cat) | Conv a (%) |
|---|---|---|---|
| 1 | 1 | 500:1 | 3 |
| 2 b | 1 | 500:1 | 2 |
| 3 | 2 | 500:1 | 3 |
| 4 | 3 | 500:1 | 1 |
| 5 | 4 | 500:1 | 10 |
| 6 b | 4 | 500:1 | 8 |
| 7 | 5 | 500:1 | 8 |
| 8 b | 5 | 500:1 | 1 |
| 9 | 6 | 500:1 | 43 |
| 10 b | 6 | 500:1 | 54 |
| 11 | I | 500:1 | 29 |
| 12 b | I | 500:1 | 39 |
| Complex | PE Yield, g | Average Activity Kg PE/mol V Bar h | Mη × 10−3 | CH3/ 1000C | Double Bonds/ 1000C | ||
|---|---|---|---|---|---|---|---|
| 888 cm−1 | 909 cm−1 | 965 cm−1 | |||||
| 1 b | 1.4 | 2800 | 450 | 4.9 | - | 0.16 | - |
| 1 | 4.3 | 8600 | 300 | 0.6 | - | 0.10 | - |
| 4 | 0.6 | 1200 | 380 | 1.8 | 0.09 | 0.90 | 0.04 |
| 5 | 0.74 | 1500 | 360 | 1.1 | 0.11 | 0.13 | - |
| 6 | 0 | 0 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsegood, M.R.J.; Clegg, W.; Redshaw, C. Vanadium Complexes Derived from O,N,O-tridentate 6-bis(o-hydroxyalkyl/aryl)pyridines: Structural Studies and Use in the Ring-Opening Polymerization of ε-Caprolactone and Ethylene Polymerization. Catalysts 2023, 13, 988. https://doi.org/10.3390/catal13060988
Elsegood MRJ, Clegg W, Redshaw C. Vanadium Complexes Derived from O,N,O-tridentate 6-bis(o-hydroxyalkyl/aryl)pyridines: Structural Studies and Use in the Ring-Opening Polymerization of ε-Caprolactone and Ethylene Polymerization. Catalysts. 2023; 13(6):988. https://doi.org/10.3390/catal13060988
Chicago/Turabian StyleElsegood, Mark R. J., William Clegg, and Carl Redshaw. 2023. "Vanadium Complexes Derived from O,N,O-tridentate 6-bis(o-hydroxyalkyl/aryl)pyridines: Structural Studies and Use in the Ring-Opening Polymerization of ε-Caprolactone and Ethylene Polymerization" Catalysts 13, no. 6: 988. https://doi.org/10.3390/catal13060988
APA StyleElsegood, M. R. J., Clegg, W., & Redshaw, C. (2023). Vanadium Complexes Derived from O,N,O-tridentate 6-bis(o-hydroxyalkyl/aryl)pyridines: Structural Studies and Use in the Ring-Opening Polymerization of ε-Caprolactone and Ethylene Polymerization. Catalysts, 13(6), 988. https://doi.org/10.3390/catal13060988