Influence of Vacancy Defects on the Interfacial Structural and Optoelectronic Properties of ZnO/ZnS Heterostructures for Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Geometric Structure of ZnO/ZnS Heterostructures with and without Interfacial Vacancy
2.2. Electronic Properties of Perfect and Defective ZnO/ZnS Heterostructures
2.3. Interfacial Charge Transfer and Bader Charge Analysis
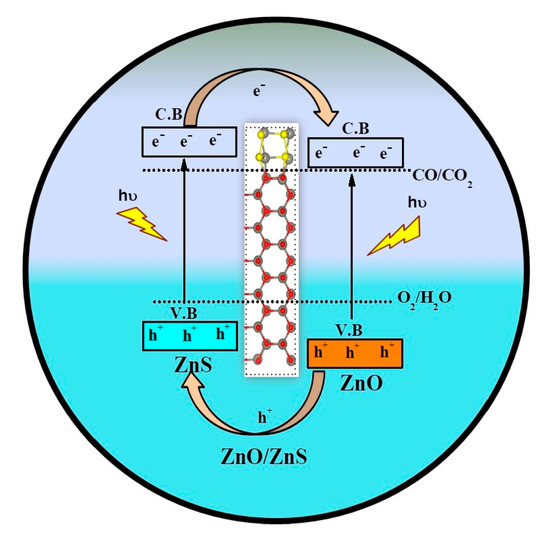
2.4. Band Alignment and Charge Separation of the Heterostructures for Photocatalysis
2.5. Optical Properties
3. Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.L.; Zhang, L.S.; Chen, Z.G.; Hu, J.Q.; Li, S.J.; Wang, Z.H.; Liu, J.S.; Wang, X.C. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; He, T. Common-cation based Z-scheme ZnS@ZnO core-shell nanostructure for efficient solar-fuel production. Appl. Catal. B 2018, 238, 518–524. [Google Scholar] [CrossRef]
- AlSalka, Y.; Osama Al-Madanat, O.; Hakki, A. TiO2-based photocatalytic hydrogen production: How to transfer it to an applicable approach. Appl. Catal. A 2023, 662, 119287. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Benitto, J.J.; Vijaya, J.J.; Bououdina, M. Recent advances in ZnO-based nanostructures for the photocatalytic degradation of hazardous, non-biodegradable medicines. Crystals 2023, 13, 329. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Morais, M.; Nunes, D.; Oliveira, M.J.; Rovisco, A.; Pimentel, A.; Aguas, H.; Fortunato, E.; Martins, R. High UV and sunlight photocatalytic performance of porous ZnO nanostructures synthesized by a facile and fast microwave hydrothermal method. Materials 2021, 14, 2385. [Google Scholar] [CrossRef]
- Ombaka, L.M.; McGettrick, J.D.; Oseghe, E.O.; Al-Madanat, O.; Best, F.R.G.; Msagati, T.A.; Davies, M.L.; Bredow, T.; Bahnemann, D.W. Photocatalytic H2 production and degradation of aqueous 2-chlorophenol over B/N-graphene-coated Cu0/TiO2: A DFT, experimental and mechanistic investigation. J. Environ. Manag. 2022, 311, 114822. [Google Scholar] [CrossRef]
- Xiao, J.-H.; Huang, W.-Q.; Hu, Y.-S.; Zeng, F.; Huang, Q.-Y.; Zhou, B.-X.; Pan, A.; Li, K.; Huang, G.-F. Facile in situ synthesis of wurtzite ZnS/ZnO core/shell heterostructure with highly efficient visible-light photocatalytic activity and photostability. J. Phys. D 2018, 51, 075501. [Google Scholar] [CrossRef]
- Flores, E.M.; Raubach, C.W.; Gouvea, R.; Longo, E.; Cava, S.; Moreira, M.L. Optical and structural investigation of ZnO@ZnS core–shell nanostructures. Mater. Chem. Phys. 2016, 173, 347–354. [Google Scholar] [CrossRef] [Green Version]
- De Moura, A.P.; Lima, R.C.; Moreira, M.L.; Volanti, D.P.; Espinosa, J.W.M.; Orlandi, M.O.; Pizani, P.S.; Varela, J.A.; Longo, E. ZnO architectures synthesized by a microwave-assisted hydrothermal method and their photoluminescence properties. Solid State Ion. 2010, 181, 775–780. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Guo, Z.Y.; Gao, X.Q.; Cao, D.X.; Dai, Y.X.; Zhao, H.T. First-principles of wurtzite ZnO (0001) and (0001) surface structures. J. Semiconduct. 2010, 31, 082001. [Google Scholar]
- Sato, K.; Bergqvist, L.; Kudrnovský, J.; Dederichs, P.H.; Eriksson, O.; Turek, I.; Sanyal, B.; Bouzerar, G.; Katayama, Y.H.; Dinh, V.A.; et al. First-principles theory of dilute magnetic semiconductors. Rev. Mod. Phys. 2010, 82, 1633–1690. [Google Scholar] [CrossRef]
- Li, P.; Hu, H.F.; Luo, G.; Zhu, S.; Guo, L.J.; Qu, P.; Shen, Q.; He, T. Crystal facet dependent CO2 photoreduction over porous ZnO nanocatalysts. ACS Appl. Mater. Interface 2020, 12, 56039–56048. [Google Scholar] [CrossRef]
- Li, R.G.; Zhang, F.X.; Wang, D.G.; Yang, J.X.; Li, M.R.; Zhu, J.; Zhou, X.; Han, H.X.; Li, C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.L.; Chang, C.J. Recent progress on metal sulfide composite nanomaterials for photocatalytic hydrogen production. Catalysts 2019, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, J.G.; Jaroniec, M.; Chen, X.B. Cocatalysts for selective photoreduction of CO2 into solar fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Yan, H.J.; Yang, J.H.; Ma, G.J.; Wu, G.P.; Zong, X.; Lei, Z.B.; Shi, J.Y.; Li, C. Visible-light-driven hydrogen production with extremely high quantum efficiency on Pt–PdS/CdS photocatalyst. J. Catal. 2009, 266, 165–168. [Google Scholar] [CrossRef]
- Yang, J.H.; Wang, D.G.; Han, H.X.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Tien, T.M.; Chen, E.L. A novel ZnO/Co3O4 nanoparticle for enhanced photocatalytic hydrogen evolution under visible light irradiation. Catalysts 2023, 13, 852. [Google Scholar] [CrossRef]
- Jin, X.K.; Chen, J.J.; Chen, F.J.; Duan, H.M.; Wang, Z.Y.; Li, J.H. Solid-state synthesis of ZnO/ZnS photocatalyst with efficient organic pollutant degradation performance. Catalysts 2022, 12, 981. [Google Scholar] [CrossRef]
- Dharmadasa, I.; Ojo, A. Unravelling complex nature of CdS/CdTe based thin film solar cells. J. Mater. Sci. Mater. Electron. 2017, 28, 16598–16617. [Google Scholar] [CrossRef] [Green Version]
- Kumazaki, Y.; Uemura, K.; Sato, T.; Hashizume, T. Precise thickness control in recess etching of AlGaN/GaN hetero-structure using photocarrier-regulated electrochemical process. J. Appl. Phys. 2017, 121, 184501. [Google Scholar] [CrossRef]
- Vaneski, A.; Schneider, J.; Susha, A.S.; Rogach, A.L. Colloidal hybrid heterostructures based on II–VI semiconductor nanocrystals for photocatalytic hydrogen generation. J. Photochem. Photobiol. C 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Abrikosov, N.K. Semiconducting II–VI, IV–VI, and V–VI Compounds; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chen, M.-M.; Xue, H.-G.; Guo, S.-P. Multinary metal chalcogenides with tetrahedral structures for second-order nonlinear optical, photocatalytic, and photovoltaic applications. Coord. Chem. Rev. 2018, 368, 115–133. [Google Scholar] [CrossRef]
- Flores, E.M.; Gouvea, R.A.; Piotrowski, M.J.; Moreira, M.L. Band alignment and charge transfer predictions of ZnO/ZnX (X = S, Se or Te) interfaces applied to solar cells: A PBE+U theoretical study. Phys. Chem. Chem. Phys. 2018, 20, 4953–4961. [Google Scholar] [CrossRef]
- Schrier, J.; Demchenko, D.O.; Alivisatos, A.P. Optical properties of ZnO/ZnS and ZnO/ZnTe heterostructures for photovoltaic applications. Nano Lett. 2007, 7, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Sadollahkhani, A.; Kazeminezhad, I.; Lu, J.; Nur, O.; Hultman, L.; Willander, M. Synthesis, structural characterization and photocatalytic application of ZnO@ZnS core-shell nanoparticles. RSC Adv. 2014, 4, 36940–36950. [Google Scholar] [CrossRef] [Green Version]
- Ranjith, K.S.; Senthamizhan, A.; Balusamy, B.; Uyar, T. Nanograined surface shell wall controlled ZnO-ZnS core-shell nanofibers and their shell wall thickness dependent visible photocatalytic properties. Catal. Sci. Technol. 2017, 7, 1167–1180. [Google Scholar] [CrossRef]
- Saha, S.; Pal, S.; Sarkar, P.; Rosa, A.; Frauenheim, T. A complete set of self-consistent charge density-functional tight-binding parametrization of zinc chalcogenides (ZnX.; X = O, S, Se, and Te). J. Comput. Chem. 2012, 33, 1165–1178. [Google Scholar] [CrossRef]
- Shannon, R.; Prewitt, C. Effective ionic radii in oxides and fluorides. Acta Crystallogr. B 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Dedova, T. Chemical Spray Pyrolysis Deposition of Zinc Sulfide Thin Films and Zinc Oxide Nanostructured Layers; Tallinn University of Technology: Tallinn, Estonia, 2007. [Google Scholar]
- Liu, J.J. Origin of high photocatalytic efficiency in monolayer g-C3N4/CdS heterostructure: A hybrid DFT study. J. Phys. Chem. C 2015, 119, 28417–28423. [Google Scholar] [CrossRef]
- Lahiri, J.; Batzill, M.J. Surface functionalization of ZnO photocatalysts with monolayer ZnS. Phys. Chem. C 2008, 112, 4304–4307. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, K.K.; van Sittert, C.G.C.E.; Govender, P.P. Insights into the photocatalytic mechanism of mediator-free direct Z-scheme g-C3N4/Bi2MoO6(010) and g-C3N4/Bi2WO6(010) heterostructures: A hybrid density functional theory study. Appl. Surf. Sci. 2018, 427B, 487–498. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Xu, J.J.; Chen, M.D.; Fu, D.G. Study on highly visible light active Bi-doped TiO2 composite hollow sphere. Appl. Surf. Sci. 2011, 257, 7381–7386. [Google Scholar] [CrossRef]
- Lin, Y.M.; Shi, H.L.; Jiang, Z.Y.; Wang, G.S.; Zhang, X.D.; Zhu, H.Y.; Zhang, R.Q.; Zhu, C.Y. Enhanced optical absorption and photocatalytic H2 production activity of g-C3N4/TiO2 heterostructure by interfacial coupling: A DFT+U study. Int. J. Hydrogen Energy 2017, 42, 9903–9913. [Google Scholar]
- Liu, Y.Y.; Lv, P.; Zhou, W.; Hong, J.W. Built-in electric field hindering photogenerated carrier recombination in polar bilayer SnO/BiOX (X = Cl, Br, I) for water splitting. J. Phys. Chem. C 2020, 124, 9696–9702. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Guo, L.J.; Louis, H.; Zhu, S.; He, T. First-principles calculations of Wurtzite ZnS1–xSex solid solutions for photocatalysis. Mater. Today Commun. 2019, 21, 100672. [Google Scholar] [CrossRef]
- Hussain, S.; Murtaza, G.; Khan, S.H.; Khan, A.; Ali, M.A.; Faizan, M.; Mahmood, A.; Khenata, R. First principles study of structural, optoelectronic and thermoelectric properties of Cu2CdSnX4 (X = S, Se, Te) chalcogenides. Mater. Res. Bull. 2016, 79, 73–83. [Google Scholar] [CrossRef]
- Luo, X.K.; Wang, G.Z.; Huang, Y.H.; Wang, B.; Yuan, H.K.; Chen, H. A two-dimensional layered CdS/C2N heterostructure for visible-light-driven photocatalysis. Phys. Chem. Chem. Phys. 2017, 19, 28216–28224. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. J. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Pham, A.; Assadi, M.H.N.; Yu, A.B.; Li, S. Critical role of Fock exchange in characterizing dopant geometry and magnetic interaction in magnetic semiconductors. Phys. Rev. B 2014, 89, 155110. [Google Scholar] [CrossRef] [Green Version]
- Sabine, M.T.; Hogg, S. The wurtzite Z parameter for beryllium oxide and zinc oxide. Acta. Crystallogr. B 1969, 25, 2254–2256. [Google Scholar] [CrossRef]
- Raubach, C.W.; de Santana, Y.V.; Ferrer, M.M.; Longo, V.M.; Varela, J.A.; Avansi, W., Jr.; Buzolin, P.G.; Sambrano, J.R.; Longo, E. Strutural and optical approach of CdS@ ZnS core-shell system. Chem. Phys. Lett. 2012, 536, 96–99. [Google Scholar] [CrossRef] [Green Version]










| a (Å) | b (Å) | c (Å) | Alpha | Beta | Gamma | Volume (Å3) | Volume Variation (%) | |
|---|---|---|---|---|---|---|---|---|
| Perfect | 5.74 | 5.37 | 24.21 | 90.00° | 90.00° | 90.00° | 746.73 | - |
| VO at interface | 5.73 | 5.33 | 24.11 | 90.04° | 90.22° | 90.40° | 736.36 | 1.4% |
| VS at interface | 5.74 | 5.35 | 23.52 | 89.85° | 90.31° | 89.94° | 723.07 | 3.1% |
| VZn at interface | 5.74 | 5.35 | 23.91 | 89.78° | 89.94° | 87.85° | 733.18 | 1.8% |
| Bond | Length (Å) | |
|---|---|---|
| Perfect | (i) O2-Zn8, (ii) Zn8-O6, (iii) O3-Zn5, (iv) Zn4-O6, (v) O6-Zn12, (vi) Zn1-O7, (vii) O7-Zn9, (viii) Zn5-O7, (ix) Zn8-S10, (x) S10-Zn16, (xi) Zn16-S15, (xii) S15-Zn9, (xiii) Zn9-S11, (xiv) Zn11-S5, (xv) S11-Zn13, (xvi) Zn12-S14, (xvii) S14-Zn16, (xviii) Zn12-S11 | (i) 2.00, (ii) 2.01, (iii) 1.99, (iv) 1.95, (v) 2.03, (vi) 1.97, (vii) 2.04, (viii) 2.04, (ix) 2.33, (x) 2.50, (xi) 2.32, (xii) 2.38, (xiii) 2.40, (xiv) 2.34, (xv) 2.37, (xvi) 2.50, (xvii) 2.31, (xviii) 2.32 |
| VO at Interface | (i) O2-Zn8, (ii) Zn8-O6, (iii) O3-Zn5, (iv) Zn4-O6, (v) O6-Zn12, (ix) Zn8-S10, (x) S10-Zn16, (xi) Zn16-S15, (xii) S15-Zn9, (xiii) Zn9-S11, (xiv) Zn11-S5, (xv) S11-Zn13, (xvi) Zn12-S14, (xvii) S14-Zn16, (xviii) Zn12-S11 | (i) 2.03, (ii) 2.05, (iii) 1.99, (iv) 2.0, (v) 2.01, (ix) 2.41, (x) 2.45, (xi) 2.33, (xii) 2.38, (xiii) 2.37, (xiv) 2.40, (xv) 2.39, (xvi) 2.48, (xvii) 2.32, (xviii) 2.36 |
| VS at Interface | (i) O2-Zn8, (ii) Zn8-O6, (iii) O3-Zn5, (iv) Zn4-O6, (v) O6-Zn12, (vi) Zn1-O7, (vii) O7-Zn9, (viii) Zn5-O7, (ix) Zn8-S10, (x) S10-Zn16, (xi) Zn16-S15, (xvi) Zn12-Zn14, (xvii) S14-Zn16, (a) S15-Zn13 | (i) 1.99, (ii) 1.98, (iii) 2.07, (iv) 2.01, (v) 2.03, (vi) 2.01, (vii) 2.05, (viii) 2.05, (ix) 2.01, (x) 2.29, (xi) 2.33, (xvi) 2.42, (xvii) 2.32, (a) 2.33 |
| VZn at interface | (i) O2-Zn8, (ii) Zn8-O6, (iv) Zn4-O6, (v) O6-Zn12, (vi) Zn1-O7, (ix) Zn8-S10, (x) S10-Zn16, (xi) Zn16-S15, (xiv) S11-Zn5, (xv) S11-Zn13, (xviii) S11-Zn12 | (i) 1.97, (ii) 1.99, (iv) 2.04, (v) 1.98, (vi) 1.95, (ix) 2.65, (x) 2.60, (xi) 2.30, (xiv) 2.39, (xv) 2.54, (xviii) 2.41 |
| Atoms | Serial No | Total Bader Charge on the Atom (e) | |
|---|---|---|---|
| Perfect | Zn | 1, 4, 5, 8, 9, 12, 13, 16 | −1.22, −1.20, −1.13, −1.11, −0.99, −0.99, −0.99, −0.99 |
| S | 10, 11, 14, 15 | 0.90, 0.92, 0.91, 0.92 | |
| O | 2, 3, 6, 7 | 1.21, 1.20, 1.21, 1.19 | |
| VO at interface | Zn | 1, 4, 5, 8, 9, 12, 13, 16 | −0.88, −1.19, −0.80, −0.74, −0.78, −0.99, −0.99, −0.99 |
| S | 10, 11, 14, 15 | 0.88, 0.91, 0.91, 0.93 | |
| O | 2, 3, 6 | 1.20, 1.18, 1.19 | |
| VS at interface | Zn | 1, 4, 5, 8, 9, 12, 13, 16 | −1.19, −1.20, −0.93, −1.11, −0.72, −0.57, −0.87, −0.97 |
| S | 10, 14, 15 | 0.87, 0.88, 0.89 | |
| O | 2, 3, 6, 7 | 1.19, 1.195, 1.187, 1.19 | |
| VZn at interface | Zn | 1, 4, 5, 8, 12, 13, 16 | −1.21, −1.19, 1.14, 1.11, −0.97, −0.98, −0.97 |
| S | 10, 11, 14, 15 | 0.42, 0.49, 0.90, 0.87 | |
| O | 2, 3, 6, 7 | 1.20, 1.19, 1.20, 1.14 |
| mh | me | r = me/mh | |
|---|---|---|---|
| Perfect | 0.470 | 0.143 | 3.30 |
| VO at interface | 0.886 | 0.277 | 3.20 |
| VS at interface | 0.367 | 0.273 | 1.34 |
| VZn at interface | 1.04 | 0.192 | 5.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Guo, L.; He, T. Influence of Vacancy Defects on the Interfacial Structural and Optoelectronic Properties of ZnO/ZnS Heterostructures for Photocatalysis. Catalysts 2023, 13, 1199. https://doi.org/10.3390/catal13081199
Hussain S, Guo L, He T. Influence of Vacancy Defects on the Interfacial Structural and Optoelectronic Properties of ZnO/ZnS Heterostructures for Photocatalysis. Catalysts. 2023; 13(8):1199. https://doi.org/10.3390/catal13081199
Chicago/Turabian StyleHussain, Sajjad, Lingju Guo, and Tao He. 2023. "Influence of Vacancy Defects on the Interfacial Structural and Optoelectronic Properties of ZnO/ZnS Heterostructures for Photocatalysis" Catalysts 13, no. 8: 1199. https://doi.org/10.3390/catal13081199
APA StyleHussain, S., Guo, L., & He, T. (2023). Influence of Vacancy Defects on the Interfacial Structural and Optoelectronic Properties of ZnO/ZnS Heterostructures for Photocatalysis. Catalysts, 13(8), 1199. https://doi.org/10.3390/catal13081199