Sulfonic Resins as Catalysts for the Oxidation of Alcohols with H2O2/KBr
Abstract
:1. Introduction
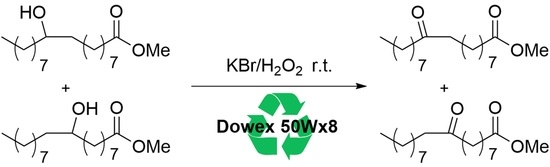
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A Review on the Chemistry, Production, and Technological Potential of Bio-Based Lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Ronda, J.C.; Lligadas, G.; Galià, M.; Cádiz, V. A Renewable Approach to Thermosetting Resins. React. Funct. Polym. 2013, 73, 381–395. [Google Scholar] [CrossRef]
- Lebarbé, T.; Grau, E.; Gadenne, B.; Alfos, C.; Cramail, H. Synthesis of Fatty Acid-Based Polyesters and Their Blends with Poly(l-Lactide) as a Way To Tailor PLLA Toughness. ACS Sustain. Chem. Eng. 2015, 3, 283–292. [Google Scholar] [CrossRef]
- Metzger, J.O.; Meier, M.A.R. Fats and Oils as Renewable Feedstock for the Chemical Industry. Eur. J. Lipid Sci. Technol. 2011, 113, 1–2. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Herrerías, C.I.; Pires, E. Synthetic Transformations for the Valorization of Fatty Acid Derivatives. Synthesis 2017, 49, 1444–1460. [Google Scholar] [CrossRef]
- Dorado, V.; Gil, L.; Mayoral, J.A.; Herrerías, C.I.; Fraile, J.M. Synthesis of Fatty Ketoesters by Tandem Epoxidation—Rearrangement with Heterogeneous Catalysis. Catal. Sci. Technol. 2020, 10, 1789–1795. [Google Scholar] [CrossRef]
- Dorado, V.; Herrerías, C.I.; Fraile, J.M. Synthesis of Hydroxyfatty Esters by Sequential Epoxidation-Hydrogenolysis: Solvent Effects. Appl. Catal. A Gen. 2021, 623, 118270. [Google Scholar] [CrossRef]
- Dorado, V.; Herrerías, C.I.; Fraile, J.M. Catalytic Hydrolysis of Epoxyfatty Esters with Solid Sulfonic Acids. Mol. Catal. 2023, 547, 113282. [Google Scholar] [CrossRef]
- Dorado, V.; Herrerías, C.I.; Fraile, J.M. Simple Metal-Free Oxidative Cleavage of 1,2-Diols. Tetrahedron 2023, 139, 133450. [Google Scholar] [CrossRef]
- Tojo, G.; Fernández, M. Oxidation of Alcohols to Aldehydes and Ketones; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 9780387236070. [Google Scholar]
- Fore, S.P.; Bickford, W.G. Catalytic Hydrogenation of Cis-6,7-Epoxyoctadecanoic Acid. J. Org. Chem. 1961, 26, 2104–2105. [Google Scholar] [CrossRef]
- Howton, D.R.; Kaiser, R.W. Silicic Acid Chromatographic Study of the Catalytic Hydrogenation Products of 9,10-Epoxystearates. J. Org. Chem. 1964, 29, 2420–2425. [Google Scholar] [CrossRef]
- Mancuso, A.J.; Huang, S.L.; Swern, D. Oxidation of Long-Chain and Related Alcohols to Carbonyls by Dimethyl Sulfoxide “Activated” by Oxalyl Chloride. J. Org. Chem. 1978, 43, 2480–2482. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Catalytic Oxidation of Alcohols: Recent Advances. Adv. Organomet. Chem. 2015, 63, 91–174. [Google Scholar] [CrossRef]
- John, L.C.; Gunay, A.; Wood, A.J.; Emmert, M.H. Catalysts for Convenient Aerobic Alcohol Oxidations in Air: Systematic Ligand Studies in Pd/Pyridine Systems. Tetrahedron 2013, 69, 5758–5764. [Google Scholar] [CrossRef]
- Giachi, G.; Frediani, M.; Oberhauser, W.; Passaglia, E. Aerobic Alcohol Oxidation Catalyzed by Polyester-Based Pd(II) Macrocomplexes. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2725–2731. [Google Scholar] [CrossRef]
- da Silva, J.A.L.; da Silva, J.J.R.F.; Pombeiro, A.J.L. Oxovanadium Complexes in Catalytic Oxidations. Coord. Chem. Rev. 2011, 255, 2232–2248. [Google Scholar] [CrossRef]
- Reddy, S.R.; Das, S.; Punniyamurthy, T. Polyaniline Supported Vanadium Catalyzed Aerobic Oxidation of Alcohols to Aldehydes and Ketones. Tetrahedron Lett. 2004, 45, 3561–3564. [Google Scholar] [CrossRef]
- Taketoshi, A.; Beh, X.N.; Kuwabara, J.; Koizumi, T.A.; Kanbara, T. Aerobic Oxidative Dehydrogenation of Benzyl Alcohols to Benzaldehydes by Using a Cyclometalated Ruthenium Catalyst. Tetrahedron Lett. 2012, 53, 3573–3576. [Google Scholar] [CrossRef]
- Fei, H.; Shin, J.; Meng, Y.S.; Adelhardt, M.; Sutter, J.; Meyer, K.; Cohen, S.M. Reusable Oxidation Catalysis Using Metal-Monocatecholato Species in a Robust Metal-Organic Framework. J. Am. Chem. Soc. 2014, 136, 4965–4973. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Lasri, J.; Guedes Da Silva, M.F.C.; Da Silva, J.A.L.; Fraústo Da Silva, J.J.R.; Pombeiro, A.J.L. Bis- and Tris-Pyridyl Amino and Imino Thioether Cu and Fe Complexes. Thermal and Microwave-Assisted Peroxidative Oxidations of 1-Phenylethanol and Cyclohexane in the Presence of Various N-Based Additives. J. Mol. Catal. A Chem. 2011, 351, 100–111. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives. Org. Process Res. Dev. 2010, 14, 245–251. [Google Scholar] [CrossRef]
- Anelli, P.L.; Biffi, C.; Montanari, F.; Quici, S. Fast and Selective Oxidation of Primary Alcohols to Aldehydes or to Carboxylic Acids and of Secondary Alcohols to Ketones Mediated by Oxoammonium Salts under Two-Phase Conditions. J. Org. Chem. 1987, 52, 2559–2562. [Google Scholar] [CrossRef]
- Moriyama, K.; Takemura, M.; Togo, H. Selective Oxidation of Alcohols with Alkali Metal Bromides as Bromide Catalysts: Experimental Study of the Reaction Mechanism. J. Org. Chem. 2014, 79, 6094–6104. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, J.P. Hydrolysis of Esters of Oxy Acids: PKa Values for Strong Acids; Bronsted Relationship for Attack of Water at Methyl; Free Energies of Hydrolysis of Esters of Oxy Acids; and a Linear Relationship between Free Energy of Hydrolysis and PKa Holding over a Ran. Can. J. Chem. 1978, 56, 2342–2353. [Google Scholar] [CrossRef]
- Li, T.; Shen, J.; Chen, G.; Guo, S.; Xie, G. Performance Comparison of Proton Exchange Membrane Fuel Cells with Nafion and Aquivion Perfluorosulfonic Acids with Different Equivalent Weights as the Electrode Binders. ACS Omega 2020, 5, 17628–17636. [Google Scholar] [CrossRef]
- Elliott, J.A.; James, P.J.; McMaster, T.J.; Newton, J.M.; Elliott, A.M.S.; Hanna, S.; Miles, M.J. Hydrolysis of the Nafion® Precursor Studied by X-Ray Scattering and in-Situ Atomic Force Microscopy. E-Polymers 2001, 1, 1–11. [Google Scholar] [CrossRef]
- Komagawa, H.; Maejima, Y.; Nagano, T. Sodium Bromide-Catalyzed Oxidation of Secondary Benzylic Alcohols Using Aqueous Hydrogen Peroxide as Terminal Oxidant. Synlett 2016, 27, 789–793. [Google Scholar] [CrossRef]
- Fraile, J.M.; García-Bordejé, E.; Pires, E.; Roldán, L. Catalytic Performance and Deactivation of Sulfonated Hydrothermal Carbon in the Esterification of Fatty Acids: Comparison with Sulfonic Solids of Different Nature. J. Catal. 2015, 324, 107–118. [Google Scholar] [CrossRef]
- Nikishin, G.I.; Sokova, L.L.; Terent’ev, A.O.; Kapustina, N.I. Primary Alkanols: Oxidative Homocondensation in Water and Cross-Condensation in Methanol. Russ. Chem. Bull. 2015, 64, 2845–2850. [Google Scholar] [CrossRef]
- Farshadfar, K.; Bird, M.J.; Olivier, W.J.; Hyland, C.J.T.; Smith, J.A.; Ariafard, A. Computational Investigation into the Mechanistic Features of Bromide-Catalyzed Alcohol Oxidation by PhIO in Water. J. Org. Chem. 2021, 86, 2998–3007. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Aquivion PW79S. Available online: https://www.fuelcellstore.com/solvay-aquivion-pw79s-72800020 (accessed on 10 January 2024).
- Nafion. Available online: https://www.nafion.com/en/products/resins (accessed on 10 January 2024).
- Aquivion P98. Available online: https://www.fuelcellstore.com/solvay-aquivion-p98-so2f-72800018 (accessed on 10 January 2024).
- Amberlyst 15. Available online: https://www.sigmaaldrich.com/ES/en/product/sial/216380 (accessed on 10 January 2024).
- Dowex 50Wx8. Available online: https://www.sigmaaldrich.com/ES/en/product/sial/217506 (accessed on 10 January 2024).
- Dowex 50Wx2. Available online: https://www.sigmaaldrich.com/ES/en/product/sial/217468 (accessed on 10 January 2024).





| Entry | Sulfonic Solid | mol% | mg | t (h) | Conversion (%) 2 |
|---|---|---|---|---|---|
| 1 | Aquivion PW79S | 20 | 39.4 | 24 | 66 |
| 48 | 81 | ||||
| 2 | Nafion NR50 | 20 | 112.4 | 24 | 56 |
| 48 | 72 | ||||
| 3 | Aquivion P98 | 20 | 102.0 | 6 | 54 |
| 24 | 90 | ||||
| 4 | Amberlyst 15 | 20 | 21.7 | 6 | 52 |
| 24 | >99 | ||||
| 5 | Dowex 50W×8 | 20 | 22.2 | 6 | 75 |
| 24 | >99 | ||||
| 6 | Dowex 50W×2 | 20 | 22.2 | 24 | 96 |
| 7 | Dowex 50W×2 | 10 | 11.1 | 24 | 49 |
| 48 | 53 | ||||
| 8 | Triflic acid | 10 | 7.5 | 24 | 97 |
| 9 | p-Toluenesulfonic acid | 20 | 17.2 | 24 | 98 |
| 10 | Dowex 50W×8 3 | 20 | 22.2 | 24 | 26 |
| 48 | 44 | ||||
| 11 | Dowex 50W×8 4 | 20 | 22.2 | 48 | 16 |
| Entry | Sulfonic Solid | Run | Conversion (%) 2 |
|---|---|---|---|
| 1 | Amberlyst 15 | 1 | >99 |
| 2 | 2 | 63 | |
| 3 | Dowex 50W×8 | 1 | >99 |
| 4 | 2 | 62 | |
| 5 | 3 3 | >99 |
| Entry | mol% Dowex 50W×8 | mol% KBr | t (h) | Conversion (%) 2 |
|---|---|---|---|---|
| 1 | 20 | 20 | 6 | 75 |
| 24 | >99 | |||
| 2 | 20 | 10 | 24 | 76 |
| 48 | 86 | |||
| 3 | 40 | 20 | 6 | 76 |
| 24 | >99 |
| Entry | Alcohol | KBr mol% | H2O2/Alcohol Molar Ratio | t (h) | Reaction Mixture Composition (%) 2 |
|---|---|---|---|---|---|
| 1 | 1-Dodecanol (3a) | 20 | 0.75 | 48 | 3a (49) + 4a (4) + 5a (47) |
| 2 | 1-Dodecanol (3a) | 20 | 2.0 | 96 | 3a (18) + 4a (8) + 5a (74) |
| 3 | 1-Dodecanol (3a) | 40 | 1.5 | 48 | 3a (12) + 4a (8) + 5a (80) |
| 4 | 1-Octadecanol (3b) | 40 | 1.5 | 48 | 3b (34) + 4b (13) + 5b (53) |
| 5 | 2-Methylcyclohexanol (6) 3 | 20 | 0.75 | 24 | 6 (3) + 7 (85) + unk (12) |
| 6 | (−)-Menthol (8) | 20 | 0.75 | 48 | 8 (36) + 9 (59) + unk (5) |
| 7 | (−)-Menthol (8) | 20 | 1.5 | 48 | 8 (17) + 9 (74) + unk (9) |
| Sulfonic Solid | Physical Form | Particle Size (mm) | Acid Functionalization 1 | |||
|---|---|---|---|---|---|---|
| Supplier | Dry 2 | Swollen 2 | Supplier | Experim. 3 | ||
| Aquivion PW79S [33] | Powder | n.r. | n.d. | n.d. | 1.23 to 1.30 | 1.26 |
| Nafion NR50 [34] | Pellets | 3 × 4 | ≈3 | ≈4 | >0.92 | 0.89 |
| Aquivion P98 [35] | Pellets | n.r. | ≈2 | ≈3.5 to 4 | 0.98 to 1.06 | 0.98 |
| Amberlyst 15 [36] | Beads | <0.3 | ≈0.5 | ≈0.8 | 4.7 | 4.40 |
| Dowex 50W×8 [37] | Beads | 0.075 to 0.15 | n.d. | n.d. | (1.7) 4 | 4.46 |
| Dowex 50W×2 [38] | Beads | (0.6) 5 | 4.45 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorado, V.; Herrerías, C.I.; Fraile, J.M. Sulfonic Resins as Catalysts for the Oxidation of Alcohols with H2O2/KBr. Catalysts 2024, 14, 74. https://doi.org/10.3390/catal14010074
Dorado V, Herrerías CI, Fraile JM. Sulfonic Resins as Catalysts for the Oxidation of Alcohols with H2O2/KBr. Catalysts. 2024; 14(1):74. https://doi.org/10.3390/catal14010074
Chicago/Turabian StyleDorado, Vicente, Clara I. Herrerías, and José M. Fraile. 2024. "Sulfonic Resins as Catalysts for the Oxidation of Alcohols with H2O2/KBr" Catalysts 14, no. 1: 74. https://doi.org/10.3390/catal14010074
APA StyleDorado, V., Herrerías, C. I., & Fraile, J. M. (2024). Sulfonic Resins as Catalysts for the Oxidation of Alcohols with H2O2/KBr. Catalysts, 14(1), 74. https://doi.org/10.3390/catal14010074