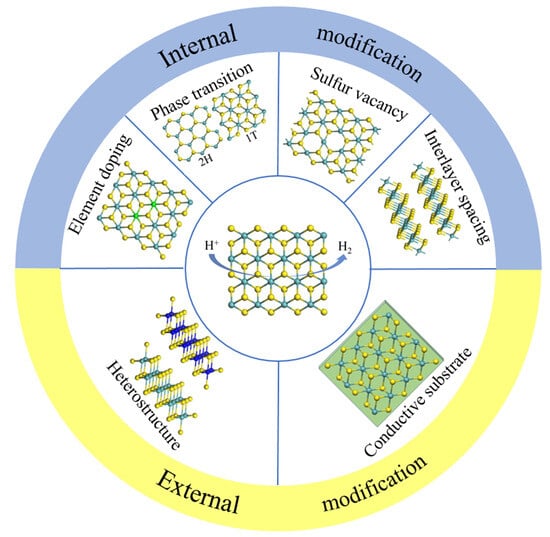
Recent Modification Strategies of MoS2 towards Electrocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Internal Modification
2.1. Interlayer Spacing
2.2. Sulfur Vacancy
2.3. Phase Transition
2.4. Element Doping
2.4.1. Metal Doping
2.4.2. Nonmetal Doping
3. External Modification
3.1. Heterostructure
3.2. Conductive Substrate
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Feng, C.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Non-noble Metal Electrocatalysts for the Hydrogen Evolution Reaction in Water Electrolysis. Electrochem. Energy Rev. 2021, 4, 473–507. [Google Scholar] [CrossRef]
- Xiao, F.X.; Miao, J.; Tao, H.B.; Hung, S.F.; Wang, H.Y.; Yang, H.B.; Chen, J.; Chen, R.; Liu, B. One-dimensional hybrid nanostructures for heterogeneous photocatalysis and photoelectrocatalysis. Small 2015, 11, 2115–2131. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, H.; Zhu, M.; Li, Y.; Li, W. Interfacial Charge Transport in 1D TiO2 Based Photoelectrodes for Photoelectrochemical Water Splitting. Small 2021, 17, 1903378. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Leng, D.; Zhang, X.; Fu, J.; Pi, C.; Zheng, Y.; Gao, B.; Li, X.; Li, N.; Chu, P.K.; et al. Enhanced Activities in Alkaline Hydrogen and Oxygen Evolution Reactions on MoS2 Electrocatalysts by In-Plane Sulfur Defects Coupled with Transition Metal Doping. Small 2022, 18, 2203173. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, G.; Ke, X.; Chen, X.; Chen, X.; Wang, Y.; Huang, G.; Dong, J.; Chu, S.; Sui, M. Direct Synthesis of Stable 1T-MoS2 Doped with Ni Single Atoms for Water Splitting in Alkaline Media. Small 2022, 18, 2107238. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yu, X.Y.; Lou, X.W. Carbon-Incorporated Nickel-Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution. Angew. Chem. Int. Ed. 2017, 56, 3897–3900. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, X.; Schwingenschlogl, U.; Alshareef, H.N. Plasma-Assisted Synthesis of NiCoP for Efficient Overall Water Splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef]
- Norskov, J.K.; Christensen, C.H. Chemistry. Toward efficient hydrogen production at surfaces. Science 2006, 312, 1322–1323. [Google Scholar] [CrossRef]
- Zhang, Z.; Cong, L.; Yu, Z.; Qu, L.; Huang, W. Facile synthesis of Fe–Ni bimetallic N-doped carbon framework for efficient electrochemical hydrogen evolution reaction. Mater. Today Energy 2020, 16, 100387. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Liu, P.; Liu, S.; Dong, R.; Zhuang, X.; Chen, M.; Feng, X. Engineering water dissociation sites in MoS2 nanosheets for accelerated electrocatalytic hydrogen production. Energy Environ. Sci. 2016, 9, 2789–2793. [Google Scholar] [CrossRef]
- Dai, X.; Du, K.; Li, Z.; Liu, M.; Ma, Y.; Sun, H.; Zhang, X.; Yang, Y. Co-Doped MoS2 Nanosheets with the Dominant CoMoS Phase Coated on Carbon as an Excellent Electrocatalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 27242–27253. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, X.; Wang, W.; Huang, Y.; Jiang, Q.; Li, W.; Chen, Y.; Yang, Y.; Li, C. Creating Edge Sites within the Basal Plane of a MoS2 Catalyst for Substantially Enhanced Hydrodeoxygenation Activity. ACS Catal. 2021, 12, 8–17. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Jin, Q.; Yun, J.; Liang, X. MoS2–Co3S4 hollow polyhedrons derived from ZIF-67 towards hydrogen evolution reaction and hydrodesulfurization. Int. J. Hydrog. Energy 2019, 44, 24246–24255. [Google Scholar] [CrossRef]
- Gao, G.; Jiao, Y.; Ma, F.; Jiao, Y.; Waclawik, E.; Du, A. Charge Mediated Semiconducting-to-Metallic Phase Transition in Molybdenum Disulfide Monolayer and Hydrogen Evolution Reaction in New 1T’ Phase. J. Phys. Chem. C 2015, 119, 13124–13128. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Wu, X.; Song, L. Gram-Scale Aqueous Synthesis of Stable Few-Layered 1T-MoS2: Applications for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.R.; Gao, D.; Ding, J.; Chao, D.; Wang, J. TMD-based highly efficient electrocatalysts developed by combined computational and experimental approaches. Chem. Soc. Rev. 2018, 47, 4332–4356. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, N.; Chen, B.; Mei, D. Mechanisms of Semiconducting 2H to Metallic 1T Phase Transition in Two-dimensional MoS2 Nanosheets. J. Phys. Chem. C 2018, 122, 28215–28224. [Google Scholar] [CrossRef]
- Benson, E.E.; Zhang, H.; Schuman, S.A.; Nanayakkara, S.U.; Bronstein, N.D.; Ferrere, S.; Blackburn, J.L.; Miller, E.M. Balancing the Hydrogen Evolution Reaction, Surface Energetics, and Stability of Metallic MoS2 Nanosheets via Covalent Functionalization. J. Am. Chem. Soc. 2018, 140, 441–450. [Google Scholar] [CrossRef]
- Venkateshwaran, S.; Senthil Kumar, S.M. Provoking Metallic 1T Phase Conversion of 2H-MoS2 via an Effectual Solvothermal Route for Electrocatalytic Water Reduction in Acid. ACS Sustain. Chem. Eng. 2022, 10, 5258–5267. [Google Scholar] [CrossRef]
- Cai, L.; Cheng, W.; Yao, T.; Huang, Y.; Tang, F.; Liu, Q.; Liu, W.; Sun, Z.; Hu, F.; Jiang, Y.; et al. High-Content Metallic 1T Phase in MoS2-Based Electrocatalyst for Efficient Hydrogen Evolution. J. Phys. Chem. C 2017, 121, 15071–15077. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Bao, S.; Zhang, Z.; Fei, H.; Wu, Z. Phase engineering of a multiphasic 1T/2H MoS2 catalyst for highly efficient hydrogen evolution. J. Mater. Chem. A 2017, 5, 2681–2688. [Google Scholar] [CrossRef]
- Li, L.; Qin, Z.; Ries, L.; Hong, S.; Michel, T.; Yang, J.; Salameh, C.; Bechelany, M.; Miele, P.; Kaplan, D.; et al. Role of Sulfur Vacancies and Undercoordinated Mo Regions in MoS2 Nanosheets toward the Evolution of Hydrogen. ACS Nano 2019, 13, 6824–6834. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yang, W.; Liu, Y.; Yu, Y. Engineering sulfur vacancies in basal plane of MoS2 for enhanced hydrogen evolution reaction. J. Catal. 2020, 391, 91–97. [Google Scholar] [CrossRef]
- Tsai, C.; Li, H.; Park, S.; Park, J.; Han, H.S.; Nørskov, J.K.; Zheng, X.; Abild-Pedersen, F. Electrochemical generation of sulfur vacancies in the basal plane of MoS2 for hydrogen evolution. Nat. Commun. 2017, 8, 15113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Li, R.; Yang, L.; Xiong, T.; Wu, Y.; Cao, L.; Yuan, D.; Zhou, W. Nitrogen doped MoS2 nanosheets synthesized via a low-temperature process as electrocatalysts with enhanced activity for hydrogen evolution reaction. J. Power Sources 2017, 356, 133–139. [Google Scholar] [CrossRef]
- Liu, Q.; Xue, Z.; Jia, B.; Liu, Q.; Liu, K.; Lin, Y.; Liu, M.; Li, Y.; Li, G. Hierarchical Nanorods of MoS2/MoP Heterojunction for Efficient Electrocatalytic Hydrogen Evolution Reaction. Small 2020, 16, 2002482. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Xu, X.; Yang, T.; Zhang, D. Preparation of the flower-like MoS2/SnS2 heterojunction as an efficient electrocatalyst for hydrogen evolution reaction. Mol. Catal. 2020, 487, 110890. [Google Scholar] [CrossRef]
- Wu, A.; Tian, C.; Yan, H.; Jiao, Y.; Yan, Q.; Yang, G.; Fu, H. Hierarchical MoS2@MoP core-shell heterojunction electrocatalysts for efficient hydrogen evolution reaction over a broad pH range. Nanoscale 2016, 8, 11052–11059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Z.L.; Dong, S.; He, D.; Lawrence, M.J.; Han, S.; Cai, C.; Xiang, S.; Rodriguez, P.; Xiang, B.; et al. Design of active nickel single-atom decorated MoS2 as a pH-universal catalyst for hydrogen evolution reaction. Nano Energy 2018, 53, 458–467. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, J.; Zhang, J.; Xi, P.; Tao, K.; Gao, D.; Xue, D. P Dopants Triggered New Basal Plane Active Sites and Enlarged Interlayer Spacing in MoS2 Nanosheets toward Electrocatalytic Hydrogen Evolution. ACS Energy Lett. 2017, 2, 745–752. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, N.; Dai, C.; Xu, R.; Wu, B.; Yu, G.; Chen, B.; Du, Y. H2-Directing Strategy on In Situ Synthesis of Co-MoS2 with Highly Expanded Interlayer for Elegant HER Activity and its Mechanism. Adv. Energy Mater. 2020, 10, 2000291. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Wang, L.; Ouyang, C.; Liang, H.; Zhong, S. A Metal-Organic Frameworks Derived 1T-MoS2 with Expanded Layer Spacing for Enhanced Electrocatalytic Hydrogen Evolution. Small 2023, 19, 2205736. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, H.; Dong, W.; Akif Munir, H.; Fan, X.; Tian, X.; Pang, L. Ammonium ions intercalated 1T/2H-MoS2 with increased interlayer spacing for high-efficient electrocatalytic hydrogen evolution reaction. J. Electroanal. Chem. 2023, 949, 117882. [Google Scholar] [CrossRef]
- Gao, M.R.; Chan, M.K.; Sun, Y. Edge-terminated molybdenum disulfide with a 9.4-A interlayer spacing for electrochemical hydrogen production. Nat. Commun. 2015, 6, 7493. [Google Scholar] [CrossRef]
- Bui, H.T.; Linh, D.C.; Nguyen, L.D.; Chang, H.; Patil, S.A.; Shrestha, N.K.; Bui, K.X.; Bui, T.S.; Nguyen, T.N.A.; Tung, N.T.; et al. In-situ formation and integration of graphene into MoS2 interlayer spacing: Expansion of interlayer spacing for superior hydrogen evolution reaction in acidic and alkaline electrolyte. J. Mater. Sci. 2022, 57, 18993–19005. [Google Scholar] [CrossRef]
- Lu, A.Y.; Yang, X.; Tseng, C.C.; Min, S.; Lin, S.H.; Hsu, C.L.; Li, H.; Idriss, H.; Kuo, J.L.; Huang, K.W.; et al. High-Sulfur-Vacancy Amorphous Molybdenum Sulfide as a High Current Electrocatalyst in Hydrogen Evolution. Small 2016, 12, 5530–5537. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Lu, A.-Y.; Tseng, C.-C.; Yang, X.; Hedhili, M.N.; Chen, M.-C.; Wei, K.-H.; Li, L.-J. Activating basal-plane catalytic activity of two-dimensional MoS2 monolayer with remote hydrogen plasma. Nano Energy 2016, 30, 846–852. [Google Scholar] [CrossRef]
- Gu, C.; Sun, T.; Wang, Z.; Jiang, S.; Wang, Z. High Resolution Electrochemical Imaging for Sulfur Vacancies on 2D Molybdenum Disulfide. Small Methods 2023, 7, 2201529. [Google Scholar] [CrossRef] [PubMed]
- Man, P.; Jiang, S.; Leung, K.H.; Lai, K.H.; Guang, Z.; Chen, H.; Huang, L.; Chen, T.; Gao, S.; Peng, Y.K.; et al. Salt-Induced High-Density Vacancy-Rich 2D MoS2 for Efficient Hydrogen Evolution. Adv. Mater. 2023, 2023, 2304808. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gong, Y.; Lin, J.; Li, B.; He, Y.; Pantelides, S.T.; Zhou, W.; Vajtai, R.; Ajayan, P.M. Defects Engineered Monolayer MoS2 for Improved Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, M.V.; Jacobsen, K.W.; Nørskov, J.K. Atomic and electronic structure of MoS2 nanoparticles. Phys. Rev. B 2003, 67, 085410. [Google Scholar] [CrossRef]
- Vojvodic, A.; Hinnemann, B.; Nørskov, J.K. Magnetic edge states in MoS2 characterized using density-functional theory. Phys. Rev. B 2009, 80, 125416. [Google Scholar] [CrossRef]
- Ali Shah, S.; Sayyar, R.; Xu, L.; Sun, H.; Khan, I.; Guo, J.; Shen, X.; Hussain, S.; Yuan, A.; Ullah, H. In-situ synthesis of NiS2 nanoparticles/MoS2 nanosheets hierarchical sphere anchored on reduced graphene oxide for enhanced electrocatalytic hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 624, 150–159. [Google Scholar] [CrossRef]
- Ali Shah, S.; Xu, L.; Sayyar, R.; Khan, I.; Yuan, A.; Shen, X.; Li, X.; Ullah, H. FeNi@N-Doped Graphene Core–Shell Nanoparticles on Carbon Matrix Coupled with MoS2 Nanosheets as a Competent Electrocatalyst for Efficient Hydrogen Evolution Reaction. Adv. Mater. Interfaces 2022, 9, 2201040. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jorgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Norskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.-E. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Hu, Y.; Zhou, X.; Zhang, M.; Jia, X.; Yang, Y.; Lin, B.-L.; Chen, G. One-Step Synthesis of 1T MoS2 Hierarchical Nanospheres for Electrocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2022, 5, 11705–11712. [Google Scholar] [CrossRef]
- Hong, Z.; Hong, W.; Wang, B.; Cai, Q.; He, X.; Liu, W. Stable 1T–2H MoS2 heterostructures for efficient electrocatalytic hydrogen evolution. Chem. Eng. J. 2023, 460, 141858. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, L.; Chen, T.; Zhou, W.; Lou, X.W. Surface Modulation of Hierarchical MoS2 Nanosheets by Ni Single Atoms for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2018, 28, 1807086. [Google Scholar] [CrossRef]
- Luo, Z.; Ouyang, Y.; Zhang, H.; Xiao, M.; Ge, J.; Jiang, Z.; Wang, J.; Tang, D.; Cao, X.; Liu, C.; et al. Chemically activating MoS2 via spontaneous atomic palladium interfacial doping towards efficient hydrogen evolution. Nat. Commun. 2018, 9, 2120. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.R.; Xu, W.X.; Wang, C.; Wang, F.B.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef]
- Sundara Venkatesh, P.; Kannan, N.; Ganesh Babu, M.; Paulraj, G.; Jeganathan, K. Transition metal doped MoS2 nanosheets for electrocatalytic hydrogen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 37256–37263. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Z.; Wei, W.; Chang, G.; Xie, Z.; Guo, W.; Liu, D.; Qu, D.; Tang, H.; Li, J. Tuning the Intrinsic Activity and Electrochemical Surface Area of MoS2 via Tiny Zn Doping: Toward an Efficient Hydrogen Evolution Reaction (HER) Catalyst. Chem. Eur. J. 2021, 27, 15992–15999. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, W.; Hu, Y.; Zhang, Y.; Dang, J.; Wu, Y.; Chen, B.; Zhao, H.; Li, Z. Single atom Ru doping 2H-MoS2 as highly efficient hydrogen evolution reaction electrocatalyst in a wide pH range. Appl. Catal. B Environ. 2021, 298, 120490. [Google Scholar] [CrossRef]
- Pei, L.; Qiao, H.; Chen, B.; Zhu, X.; Davis, R.A.; Zhu, K.; Xia, L.; Dong, P.; Ye, M.; Shen, J. Pt Edge-Doped MoS2: Activating the Active Sites for Maximized Hydrogen Evolution Reaction Performance. Small 2021, 17, 2104245. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, B.; Peng, W.; Wang, C.; Li, K.; Zhu, Y.; Mei, Y. A palladium doped 1T-phase molybdenum disulfide–black phosphorene two-dimensional van der Waals heterostructure for visible-light enhanced electrocatalytic hydrogen evolution. Nanoscale 2021, 13, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Hao, X.; Zhou, J.; Song, D.; Wang, D.; Hou, L.; Gao, F. Fluorine- and Nitrogen-Codoped MoS2 with a Catalytically Active Basal Plane. ACS Appl. Mater. Interfaces 2017, 9, 27715–27719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, M.; Yang, H.; Li, G.; Xing, S.; Li, M.; Xu, Y.; Zhang, Q.; Hu, S.; Liao, H.; et al. Creating Fluorine-Doped MoS2 Edge Electrodes with Enhanced Hydrogen Evolution Activity. Small Methods 2021, 5, 2100612. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yang, C.; Hao, R.; Li, F.; Liu, Z.; Chen, W.; Lv, Y.; Lin, C. Fabrication of phosphorus-mediated MoS2 nanosheets on carbon cloth for enhanced hydrogen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 17871–17878. [Google Scholar] [CrossRef]
- Yin, Y.; Han, J.; Zhang, Y.; Zhang, X.; Xu, P.; Yuan, Q.; Samad, L.; Wang, X.; Wang, Y.; Zhang, Z.; et al. Contributions of Phase, Sulfur Vacancies, and Edges to the Hydrogen Evolution Reaction Catalytic Activity of Porous Molybdenum Disulfide Nanosheets. J. Am. Chem. Soc. 2016, 138, 7965–7972. [Google Scholar] [CrossRef]
- Wang, J.; Yan, M.; Zhao, K.; Liao, X.; Wang, P.; Pan, X.; Yang, W.; Mai, L. Field Effect Enhanced Hydrogen Evolution Reaction of MoS2 Nanosheets. Adv. Mater. 2017, 29, 1604464. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, L.; Shang, C.; Wang, E.; Wang, J. A Cake-Style CoS2@MoS2/RGO Hybrid Catalyst for Efficient Hydrogen Evolution. Adv. Funct. Mater. 2016, 27, 1602699. [Google Scholar] [CrossRef]
- Chen, W.; Gu, J.; Du, Y.; Song, F.; Bu, F.; Li, J.; Yuan, Y.; Luo, R.; Liu, Q.; Zhang, D. Achieving Rich and Active Alkaline Hydrogen Evolution Heterostructures via Interface Engineering on 2D 1T-MoS2 Quantum Sheets. Adv. Funct. Mater. 2020, 30, 2000551. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A Heterostructure Coupling of Exfoliated Ni-Fe Hydroxide Nanosheet and Defective Graphene as a Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Jiang, J.; Lin, W.; Zhu, S.; Sha, J.; Ma, L.; Zhao, N. In-situ synthesis of MoS2/Co9S8 heterostructure for efficient HER electrocatalyst. Mater. Lett. 2021, 292, 129621. [Google Scholar] [CrossRef]
- Hao, J.; Hu, H.; Dong, Y.; Hu, J.; Sang, X.; Duan, F.; Lu, S.; Zhu, H.; Du, M. Interface engineering in core–shell Co9S8@MoS2 nanocrystals induces enhanced hydrogen evolution in acidic and alkaline media. New J. Chem. 2021, 45, 11167–11173. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, X.; Xu, S.; Wang, K.; Yuan, S.; Li, N. Constructing a 1T-MoS2/Ni3S4 heterostructure to balance water dissociation and hydroxyl desorption for efficient hydrogen evolution. Catal. Sci. Technol. 2023, 13, 3901–3909. [Google Scholar] [CrossRef]
- Kim, M.; Anjum, M.A.R.; Choi, M.; Jeong, H.Y.; Choi, S.H.; Park, N.; Lee, J.S. Covalent 0D–2D Heterostructuring of Co9S8–MoS2 for Enhanced Hydrogen Evolution in All pH Electrolytes. Adv. Funct. Mater. 2020, 30, 2002536. [Google Scholar] [CrossRef]
- Tang, J.; Huang, J.; Ding, D.; Zhang, S.; Deng, X. Research progress of 1T-MoS2 in electrocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2022, 47, 39771–39795. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Y.; Liu, S.; Li, J.; Bu, L.; Chen, J.; Xiao, X.; Choi, J.-H.; Gao, L.; Lee, J.-M. Confined growth of pyridinic N–Mo2C sites on MXenes for hydrogen evolution. J. Mater. Chem. A 2020, 8, 7109–7116. [Google Scholar] [CrossRef]
- Li, G.; Chen, Z.; Li, Y.; Zhang, D.; Yang, W.; Liu, Y.; Cao, L. Engineering Substrate Interaction To Improve Hydrogen Evolution Catalysis of Monolayer MoS2 Films beyond Pt. ACS Nano 2020, 14, 1707–1714. [Google Scholar] [CrossRef]
- Hu, H.; Xu, J.; Zheng, Y.; Zhu, Y.; Rong, J.; Zhang, T.; Yang, D.; Qiu, F. NiS2-Coated Carbon Fiber Paper Decorated with MoS2 Nanosheets for Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 10933–10940. [Google Scholar] [CrossRef]
- Ma, W.; Li, W.; Zhang, H.; Wang, Y. N-doped carbon wrapped CoFe alloy nanoparticles with MoS2 nanosheets as electrocatalyst for hydrogen and oxygen evolution reactions. Int. J. Hydrog. Energy 2023, 48, 22032–22043. [Google Scholar] [CrossRef]

















| Strategy | Material | Electrolyte | η10 (mV) | Tafel Slope (mV dec−1) | Ref. |
|---|---|---|---|---|---|
| Interlayer spacing | 200-1T-MoS2 | 0.5 M H2SO4 | 98 | 52 | [36] |
| 1T/2H-MoS2/NH4+-200 | 0.5 M H2SO4 | 159.9 | 55.5 | [37] | |
| Co-MoS2-1.4 | 0.5 M H2SO4 | 56 | 32 | [35] | |
| 240-MoS2 | 0.5 M H2SO4 | 149 | 49 | [38] | |
| Sulfur vacancy | SV-2H-MoS2 | 0.5 M H2SO4 | 369 | 68.7 | [42] |
| MoS2-2.5 | 0.5 M H2SO4 | 90 | 54.3 | [43] | |
| SV-MoS2 | H2SO4 (pH = 0.2) | 170 | 60 | [45] | |
| Phase transition | 1T MoS2 NSP | 0.5 M H2SO4 | 188 | 58.47 | [55] |
| 1T-2H MoS2 | 0.5 M H2SO4 | 212 | 78 | [56] | |
| Metal doping | Ni-MoS2 | 0.5 M H2SO4 | 302.4 | 66.27 | [60] |
| Zn-1T/2H-MoS2 | 0.5 M H2SO4 | 190 | 58 | [61] | |
| Ru0.10@2H-MoS2 | 0.5 M H2SO4 | 168 | 77.5 | [62] | |
| Fe-1T-MoS2 | 1.0 M KOH | 269 | 168 | [6] | |
| Co-1T-MoS2 | 1.0 M KOH | 261 | 88.5 | ||
| Ni-1T-MoS2 | 1.0 M KOH | 199 | 52.7 | ||
| Pt-MoS2 | 0.5 M H2SO4 | 59 | 23.58 | [63] | |
| Pd-1T-MoS2 | 0.5 M H2SO4 | 170 | 98 | [64] | |
| Nonmetal doping | Etched MoS2 | 0.5 M H2SO4 | 267 | 65 | [66] |
| P-MoS2/CC-300 | 0.5 M H2SO4 | 81 | 98 | [67] | |
| Heterostructure | 1T-MoS2/Ni3S4/CC | 1 M KOH | 44 | 43 | [75] |
| MoS2@Co9S8/CC | 1 M KOH | 73 | 78 | [73] | |
| Conductive substrate | NiS2@MoS2/CFP | 0.5 M H2SO4 | 95 | 65 | [80] |
| MoS2/CoFe@NC | 1 M KOH | 172 | 122.4 | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liu, N.; Chen, B.; Dai, C.; Wang, N. Recent Modification Strategies of MoS2 towards Electrocatalytic Hydrogen Evolution. Catalysts 2024, 14, 126. https://doi.org/10.3390/catal14020126
Liu L, Liu N, Chen B, Dai C, Wang N. Recent Modification Strategies of MoS2 towards Electrocatalytic Hydrogen Evolution. Catalysts. 2024; 14(2):126. https://doi.org/10.3390/catal14020126
Chicago/Turabian StyleLiu, Lei, Ning Liu, Biaohua Chen, Chengna Dai, and Ning Wang. 2024. "Recent Modification Strategies of MoS2 towards Electrocatalytic Hydrogen Evolution" Catalysts 14, no. 2: 126. https://doi.org/10.3390/catal14020126
APA StyleLiu, L., Liu, N., Chen, B., Dai, C., & Wang, N. (2024). Recent Modification Strategies of MoS2 towards Electrocatalytic Hydrogen Evolution. Catalysts, 14(2), 126. https://doi.org/10.3390/catal14020126