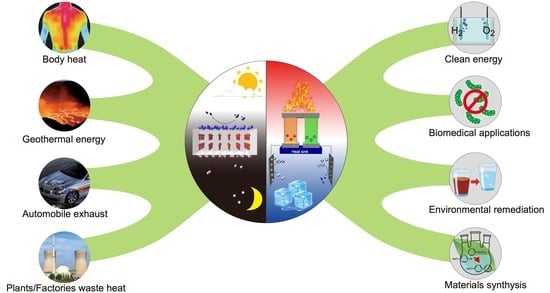
Harvesting Thermal Energy through Pyroelectric and Thermoelectric Nanomaterials for Catalytic Applications
Abstract
:1. Introduction
1.1. Waste Heat Energy
1.2. Pyroelectric and Thermoelectric Materials for Converting Waste Heat to Chemical Energy
1.3. Purpose of This Review
2. Pyroelectric Catalysis
2.1. Pyroelectric Effect
2.2. Mechanism and Key Factors of Pyroelectric Catalysis
2.3. Recent Progress in Pyroelectric Catalysis
3. Thermoelectric Catalysis Effect
3.1. Thermoelectric Effect
3.2. Thermoelectric Catalytic Effect
3.3. Recent Progress in Thermoelectric Catalysis
4. Future Prospects
- (1)
- PE catalysis.
- (2)
- TE catalysis.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Griggs, D.; Stafford-Smith, M.; Gaffney, O.; Rockström, J.; Öhman, M.C.; Shyamsundar, P.; Steffen, W.; Glaser, G.; Kanie, N.; Noble, I. Sustainable development goals for people and planet. Nature 2013, 495, 305–307. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S. Thermoelectric generators: Linking material properties and systems engineering for waste heat recovery applications. Sustain. Mater. Technol. 2014, 1–2, 26–35. [Google Scholar] [CrossRef]
- Bowen, C.R.; Taylor, J.; LeBoulbar, E.; Zabek, D.; Chauhan, A.; Vaish, R. Pyroelectric materials and devices for energy harvesting applications. Energy Environ. Sci. 2014, 7, 3836–3856. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, H.; Bowen, C.R.; Yang, Y. Recent Advances in Pyroelectric Materials and Applications. Small 2021, 17, 2103960. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, Y.; Fu, C.; Heremans, J.P.; Snyder, J.G.; Zhao, X. Compromise and Synergy in High-Efficiency Thermoelectric Materials. Adv. Mater. 2017, 29, 1605884. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Shi, X.; Zhao, L.-D.; Zou, J. High-performance SnSe thermoelectric materials: Progress and future challenge. Prog. Mater. Sci. 2018, 97, 283–346. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef]
- Bell, L.E. Cooling, Heating, Generating Power, and Recovering Waste Heat with Thermoelectric Systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, M.; Sharma, J.D.; Kumar, S.; Vaish, R. Ferroelectric ceramics for pyrocatalytic applications. Prog. Solid State Chem. 2023, 72, 100428. [Google Scholar] [CrossRef]
- de Vivanco, M.U.; Zschornak, M.; Stöcker, H.; Jachalke, S.; Mehner, E.; Leisegang, T.; Meyer, D.C. Pyroelectrically-driven chemical reactions described by a novel thermodynamic cycle. Phys. Chem. Chem. Phys. 2020, 22, 17781–17790. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Qiao, Y.; Min, M.; Wang, L.; Shan, H.; Ma, Y.; Hao, W.; Tao, P.; Shang, W.; et al. Pyroelectric Synthesis of Metal–BaTiO3 Hybrid Nanoparticles with Enhanced Pyrocatalytic Performance. ACS Sustain. Chem. Eng. 2018, 7, 2602–2609. [Google Scholar] [CrossRef]
- Schlechtweg, J.; Raufeisen, S.; Stelter, M.; Braeutigam, P. A novel model for pyro-electro-catalytic hydrogen production in pure water. Phys. Chem. Chem. Phys. 2019, 21, 23009–23016. [Google Scholar] [CrossRef]
- Wu, Z.; Lou, W.; Zhang, H.; Jia, Y. Strong pyro-catalysis of shape-controllable bismuth oxychloride nanomaterial for wastewater remediation. Appl. Sur. Sci. 2020, 513, 145630. [Google Scholar] [CrossRef]
- Xie, M.; Dunn, S.; Boulbar, E.L.; Bowen, C.R. Pyroelectric energy harvesting for water splitting. Int. J. Hydrogen Energy 2017, 42, 23437–23445. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Yao, G.; Huang, Z.; Xie, Y.; Su, Y.; Yang, W.; Zheng, C.; Lin, Y. Simultaneously Harvesting Thermal and Mechanical Energies based on Flexible Hybrid Nanogenerator for Self-Powered Cathodic Protection. ACS Appl. Mater. Interfaces 2015, 7, 28142–28147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kumar, S.; Marken, F.; Krasny, M.; Roake, E.; Eslava, S.; Dunn, S.; Da Como, E.; Bowen, C.R. Pyro-electrolytic water splitting for hydrogen generation. Nano Energy 2019, 58, 183–191. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, M.; Adamaki, V.; Khanbareh, H.; Bowen, C.R. Control of electro-chemical processes using energy harvesting materials and devices. Chem. Soc. Rev. 2017, 46, 7757–7786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Meng, Y.; Liu, Z.; Li, D.; Bai, Y.; Yuan, G.; Wang, Y.; Zhang, X.; Li, X.; et al. Pyro-catalysis for tooth whitening via oral temperature fluctuation. Nat. Commun. 2022, 13, 4419. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Jia, Y.; Wu, Z.; Wang, F.; Huang, H.; Wang, Y. Room-temperature pyro-catalytic hydrogen generation of 2D few-layer black phosphorene under cold-hot alternation. Nat. Commun. 2018, 9, 2889. [Google Scholar] [CrossRef]
- Achour, A.; Chen, K.; Reece, M.J.; Huang, Z. Tuning of Catalytic Activity by Thermoelectric Materials for Carbon Dioxide Hydrogenation. Adv. Energy Mater. 2018, 8, 1701430. [Google Scholar] [CrossRef]
- Achour, A.; Liu, J.; Peng, P.; Shaw, C.; Huang, Z. In Situ Tuning of Catalytic Activity by Thermoelectric Effect for Ethylene Oxidation. ACS Catal. 2018, 8, 10164–10172. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Khan, I.; Saha, S.; Wu, C.-C.; Barman, S.R.; Kao, F.-C.; Lin, Z.-H. Thermocatalytic hydrogen peroxide generation and environmental disinfection by Bi2Te3 nanoplates. Nat. Commun. 2021, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dong, S.; Yu, C.; Liu, J.; Gai, S.; Xie, Y.; Zhao, Z.; Qin, X.; Feng, L.; Yang, P.; et al. Mitochondria-targeting Cu3VS4 nanostructure with high copper ionic mobility for photothermoelectric therapy. Sci. Adv. 2023, 9, eadi9980. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, T.; Zhang, X.; Costin, G.; Yazdi, S.; Woellner, C.F.; Liu, Y.; Tiwary, C.S.; Ajayan, P. Thermoelectricity Enhanced Electrocatalysis. Nano Lett. 2017, 17, 7908–7913. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qiao, Y.; Liu, X.; Zhu, S.; Zheng, Y.; Jiang, H.; Zhang, Y.; Shen, J.; Li, Z.; Liang, Y.; et al. Reduced Graphene Oxides Modified Bi2Te3 Nanosheets for Rapid Photo-Thermoelectric Catalytic Therapy of Bacteria-Infected Wounds. Adv. Funct. Mater. 2023, 33, 2210098. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, M.; Fang, Y.; Yang, Z.; Dai, X.; Gu, P.; Feng, W.; Chen, Y. A Photo-Activated Thermoelectric Catalyst for Ferroptosis-/Pyroptosis-Boosted Tumor Nanotherapy. Adv. Healthc. Mater. 2023, 12, 2300699. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Z.; Zhao, J.; Zhang, Z.; Li, X.; Zhang, J. Recent Advances of Ferro-, Piezo-, and Pyroelectric Nanomaterials for Catalytic Applications. ACS Appl. Nano Mater. 2020, 3, 1063–1079. [Google Scholar] [CrossRef]
- Zhang, Y.; Phuong, P.T.T.; Roake, E.; Khanbareh, H.; Wang, Y.; Dunn, S.; Bowen, C. Thermal Energy Harvesting Using Pyroelectric-Electrochemical Coupling in Ferroelectric Materials. Joule 2020, 4, 301–309. [Google Scholar] [CrossRef]
- Wang, C.; Tian, N.; Ma, T.; Zhang, Y.; Huang, H. Pyroelectric catalysis. Nano Energy 2020, 78, 105371. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Shi, L.; Tian, N.; Huang, H.; Ou, H.; Zhang, Y. Energy and environmental catalysis driven by stress and temperature-variation. J. Mater. Chem. A 2021, 9, 12400–12432. [Google Scholar] [CrossRef]
- Achour, A. In-Situ Tuning of Catalytic Activity by Thermoelectric Effect. Ph.D. Thesis, Cranfield University, Bedford, UK, 2017. [Google Scholar]
- Xu, X.; Xiao, L.; Jia, Y.; Wu, Z.; Wang, F.; Wang, Y.; Haugen, N.O.; Huang, H. Pyro-catalytic hydrogen evolution by Ba0.7Sr0.3TiO3 nanoparticles: Harvesting cold–hot alternation energy near room-temperature. Energy Environ. Sci. 2018, 11, 2198–2207. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Lee, S.; Kim, D.; Hwang, W.; Wang, Z.L. Hybrid Energy Cell for Degradation of Methyl Orange by Self-Powered Electrocatalytic Oxidation. Nano Lett. 2013, 13, 803–808. [Google Scholar] [CrossRef]
- Wu, J.; Mao, W.; Wu, Z.; Xu, X.; You, H.; Xue, A.X.; Jia, Y. Strong pyro-catalysis of pyroelectric BiFeO3 nanoparticles under a room-temperature cold–hot alternation. Nanoscale 2016, 8, 7343–7350. [Google Scholar] [CrossRef]
- Kakekhani, A.; Ismail-Beigi, S. Polarization-driven catalysis via ferroelectric oxide surfaces. Phys. Chem. Chem. Phys. 2016, 18, 19676–19695. [Google Scholar] [CrossRef] [PubMed]
- Kakekhani, A.; Ismail-Beigi, S. Ferroelectric oxide surface chemistry: Water splitting via pyroelectricity. J. Mater. Chem. A 2016, 4, 5235–5246. [Google Scholar] [CrossRef]
- Kakekhani, A.; Ismail-Beigi, S.; Altman, E.I. Ferroelectrics: A pathway to switchable surface chemistry and catalysis. Surf. Sci. 2016, 650, 302–316. [Google Scholar] [CrossRef]
- Min, M.; Liu, Y.; Song, C.; Zhao, D.; Wang, X.; Qiao, Y.; Feng, R.; Hao, W.; Tao, P.; Shang, W.; et al. Photothermally Enabled Pyro-Catalysis of a BaTiO3 Nanoparticle Composite Membrane at the Liquid/Air Interface. ACS Appl. Mater. Interfaces 2018, 10, 21246–21253. [Google Scholar] [CrossRef]
- Qian, W.; Wu, Z.; Jia, Y.; Hong, Y.; Xu, X.; You, H.; Zheng, Y.; Xia, Y. Thermo-electrochemical coupling for room temperature thermocatalysis in pyroelectric ZnO nanorods. Electrochem. Commun. 2017, 81, 124–127. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Q.; Ma, K.; Ding, Y.; Li, C. Pyroelectric effect in CdS nanorods decorated with a molecular Co-catalyst for hydrogen evolution. Nano Energy 2020, 73, 104810. [Google Scholar] [CrossRef]
- You, H.; Li, S.; Fan, Y.; Guo, X.; Lin, Z.; Ding, R.; Cheng, X.; Zhang, H.; Lo, T.W.B.; Hao, J.; et al. Accelerated pyro-catalytic hydrogen production enabled by plasmonic local heating of Au on pyroelectric BaTiO3 nanoparticles. Nat. Commun. 2022, 13, 6144. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Jia, Y.; Hu, G.; Hu, J.; Yuan, B.; Yu, Y.; Zou, G. Pyroelectric nanoplates for reduction of CO2 to methanol driven by temperature-variation. Nat. Commun. 2021, 12, 318. [Google Scholar] [CrossRef] [PubMed]
- Dashevsky, Z.; Jarashneli, A.; Unigovski, Y.; Dzunzda, B.; Gao, F.; Shneck, R.Z. Development of a High Perfomance Gas Thermoelectric Generator (TEG) with Possibible Use of Waste Heat. Energies 2022, 15, 3960. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Shi, X.-L.; Ao, D.-W.; Liu, W.-D.; Li, M.; Kou, L.-Z.; Chen, Y.-X.; Li, F.; Wei, M.; Liang, G.-X.; et al. Harvesting waste heat with flexible Bi2Te3 thermoelectric thin film. Nat. Sustain. 2023, 6, 180–191. [Google Scholar] [CrossRef]
- Dashevsky, Z.; Skipidarov, S. Investigating the Performance of Bismuth-Antimony Telluride. In Novel Thermoelectric Materials and Device Design Concepts; Skipidarov, S., Nikitin, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–21. [Google Scholar]
- Tritt, T.M.; Subramanian, M.A. Thermoelectric Materials, Phenomena, and Applications: A Bird’s Eye View. MRS Bull. 2011, 31, 188–198. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. In Materials for Sustainable Energy; Nature Publishing Group: New York, NY, USA, 2010; pp. 101–110. [Google Scholar]
- Zhao, L.D.; Zhang, B.P.; Li, J.F.; Zhang, H.L.; Liu, W.S. Enhanced thermoelectric and mechanical properties in textured n-type Bi2Te3 prepared by spark plasma sintering. Solid State Sci. 2008, 10, 651–658. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Zhang, B.-P.; Li, J.-F.; Zhou, M.; Liu, W.-S.; Liu, J. Thermoelectric and mechanical properties of nano-SiC-dispersed Bi2Te3 fabricated by mechanical alloying and spark plasma sintering. J. Alloys Compd. 2008, 455, 259–264. [Google Scholar] [CrossRef]
- Cao, T.; Shi, X.-L.; Li, M.; Hu, B.; Chen, W.; Liu, W.-D.; Lyu, W.; MacLeod, J.; Chen, Z.-G. Advances in bismuth-telluride-based thermoelectric devices: Progress and challenges. eScience 2023, 3, 100122. [Google Scholar] [CrossRef]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef]
- Zhao, L.D.; Wu, H.J.; Hao, S.Q.; Wu, C.I.; Zhou, X.Y.; Biswas, K.; He, J.Q.; Hogan, T.P.; Uher, C.; Wolverton, C.; et al. All-scale hierarchical thermoelectrics: MgTe in PbTe facilitates valence band convergence and suppresses bipolar thermal transport for high performance. Energy Environ. Sci. 2013, 6, 3346–3355. [Google Scholar] [CrossRef]
- Chang, C.; Wang, D.; He, D.; He, W.; Zhu, F.; Wang, G.; He, J.; Zhao, L.D. Realizing High-Ranged Out-of-Plane ZTs in N-Type SnSe Crystals through Promoting Continuous Phase Transition. Adv. Energy Mater. 2019, 9, 1901334. [Google Scholar] [CrossRef]
- Qin, B.; Wang, D.; He, W.; Zhang, Y.; Wu, H.; Pennycook, S.J.; Zhao, L.-D. Realizing High Thermoelectric Performance in p-Type SnSe through Crystal Structure Modification. J. Am. Chem. Soc. 2018, 141, 1141–1149. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, D.; He, J.; Zhao, L.-D. Attempting to realize n-type BiCuSeO. J. Solid State Chem. 2018, 258, 510–516. [Google Scholar] [CrossRef]
- Zhao, L.D.; Berardan, D.; Pei, Y.L.; Byl, C.; Pinsard-Gaudart, L.; Dragoe, N. Bi1−xSrxCuSeO oxyselenides as promising thermoelectric materials. Appl. Phys. Lett. 2010, 97, 092118. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Wan, S.; Lim, K.H.; Zhang, Y.; Li, M.; Li, J.; Ibáñez, M.; Hong, M.; Cabot, A. Surface Chemistry and Band Engineering in AgSbSe2: Toward High Thermoelectric Performance. ACS Nano 2023, 17, 11923–11934. [Google Scholar] [CrossRef]
- Burmistrov, I.; Khanna, R.; Gorshkov, N.; Kiselev, N.; Artyukhov, D.; Boychenko, E.; Yudin, A.; Konyukhov, Y.; Kravchenko, M.; Gorokhovsky, A.; et al. Advances in Thermo-Electrochemical (TEC) Cell Performances for Harvesting Low-Grade Heat Energy: A Review. Sustainability 2022, 14, 9483. [Google Scholar] [CrossRef]
- Ohta, T.; Asakura, S.; Yamaguchi, M.; Kamiya, N.; Gotgh, N.; Otagawa, T. Photochemical and thermoelectric utilization of solar energy in a hybrid water-splitting system☆. Int. J. Hydrogen Energy 1976, 1, 113–116. [Google Scholar] [CrossRef]
- Ohta, T.; Kamiya, N.; Yamaguchi, M.; Gotoh, N.; Otagawa, T.; Asakura, S. System efficiency of a water-splitting system synthesized by photochemical and thermoelectric conversion of solar energy. Int. J. Hydrogen Energy 1978, 3, 203–208. [Google Scholar] [CrossRef]
- Pornrungroj, C.; Andrei, V.; Reisner, E. Thermoelectric–Photoelectrochemical Water Splitting under Concentrated Solar Irradiation. J. Am. Chem. Soc. 2023, 145, 13709–13714. [Google Scholar] [CrossRef]
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced Thermoelectric Design: From Materials and Structures to Devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef]
- He, P.; Zhang, L.; Wu, L.; Xiao, S.; Ren, X.; He, R.; Yang, X.; Liu, R.; Duan, T. Synergy of oxygen vacancies and thermoelectric effect enhances uranium(VI) photoreduction. Appl. Catal. B Environ. 2023, 322, 122087. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, Y.; Wang, H.; Zhang, J.; Zhao, M.; Huang, Z.; Wang, Y.; Zhang, L.; Deng, Y.; Hu, W.; et al. Designing Breathing Air-electrode and Enhancing the Oxygen Electrocatalysis by Thermoelectric Effect for Efficient Zn-air Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302689. [Google Scholar] [CrossRef]
- Ji, X.; Tang, Z.; Liu, H.; Kang, Y.; Chen, L.; Dong, J.; Chen, W.; Kong, N.; Tao, W.; Xie, T. Nanoheterojunction-Mediated Thermoelectric Strategy for Cancer Surgical Adjuvant Treatment and β-Elemene Combination Therapy. Adv. Mater. 2023, 35, 2207391. [Google Scholar] [CrossRef]
- Pan, C.; Ou, M.; Wang, Y.; Xiao, Q.; Mei, L.; Ji, X. p-n Heterojunction-based Thermoelectric Generator for Highly Efficient Cancer Thermoelectric Therapy. Res. Sq. 2021, preprint. [Google Scholar] [CrossRef]
- Yuan, X.; Kang, Y.; Dong, J.; Li, R.; Ye, J.; Fan, Y.; Han, J.; Yu, J.; Ni, G.; Ji, X.; et al. Self-triggered thermoelectric nanoheterojunction for cancer catalytic and immunotherapy. Nat. Commun. 2023, 14, 5140. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Z.; Li, J.; Liu, H.; Liu, M.; Zhang, Y.; Su, L.; Pérez-Jiménez, A.I.; Guo, Y.; Yang, F.; et al. Mechanically Induced Highly Efficient Hydrogen Evolution from Water over Piezoelectric SnSe nanosheets. Small 2022, 18, 2202507. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Z.; Liu, M.; Liu, X.; Huang, W.; Sun, S.; Jiang, Y.; Liu, Y.; Zhang, J.; Zhang, Z. Remarkably enhanced photocatalytic performance of Au/AgNbO3 heterostructures by coupling piezotronic with plasmonic effects. Nano Energy 2022, 95, 107031. [Google Scholar] [CrossRef]
- Zhang, Y.; Ohta, H. Electron Sandwich Doubles the Thermoelectric Power Factor of SrTiO3. Phys. Status Solidi A 2019, 216, 1800832. [Google Scholar] [CrossRef]
- Zhang, Y.; Sugo, K.; Cho, H.J.; Ohta, H. Thermoelectric phase diagram of the SrTiO3-LaTiO3 solid-solution system through a metal to Mott insulator transition. J. Appl. Phys. 2019, 126, 07510. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, B.; Hayashi, H.; Tohei, T.; Tanaka, I.; Ikuhara, Y.; Ohta, H. Thermoelectric phase diagram of the SrTiO3–SrNbO3 solid solution system. J. Appl. Phys. 2017, 121, 185102. [Google Scholar] [CrossRef]
- Hicks, L.D.; Dresselhaus, M.S. Effect of quantum-well structures on the thermoelectric figure of merit. Phys. Rev. B 1993, 47, 12727–12731. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.D.; Dresselhaus, M.S. Thermoelectric figure of merit of a one-dimensional conductor. Phys. Rev. B 1993, 47, 16631–16634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Feng, B.; Hayashi, H.; Chang, C.P.; Sheu, Y.M.; Tanaka, I.; Ikuhara, Y.; Ohta, H. Double thermoelectric power factor of a 2D electron system. Nat. Commun. 2018, 9, 2224. [Google Scholar] [CrossRef] [PubMed]







| Materials | Working Condition | Mechanism | |
|---|---|---|---|
| PE catalysis | Pyroelectric materials | Temperature fluctuation | Polarization charges |
| TE catalysis | Thermoelectric materials | Temperature gradient | Thermal-excited free charges |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, X.; Zhang, X.; Wang, Y.; Chen, S.; Liu, Y.; Zhang, Y. Harvesting Thermal Energy through Pyroelectric and Thermoelectric Nanomaterials for Catalytic Applications. Catalysts 2024, 14, 159. https://doi.org/10.3390/catal14030159
Li S, Liu X, Zhang X, Wang Y, Chen S, Liu Y, Zhang Y. Harvesting Thermal Energy through Pyroelectric and Thermoelectric Nanomaterials for Catalytic Applications. Catalysts. 2024; 14(3):159. https://doi.org/10.3390/catal14030159
Chicago/Turabian StyleLi, Shun, Xinbo Liu, Xinyue Zhang, Youling Wang, Shanliang Chen, Yong Liu, and Yuqiao Zhang. 2024. "Harvesting Thermal Energy through Pyroelectric and Thermoelectric Nanomaterials for Catalytic Applications" Catalysts 14, no. 3: 159. https://doi.org/10.3390/catal14030159
APA StyleLi, S., Liu, X., Zhang, X., Wang, Y., Chen, S., Liu, Y., & Zhang, Y. (2024). Harvesting Thermal Energy through Pyroelectric and Thermoelectric Nanomaterials for Catalytic Applications. Catalysts, 14(3), 159. https://doi.org/10.3390/catal14030159