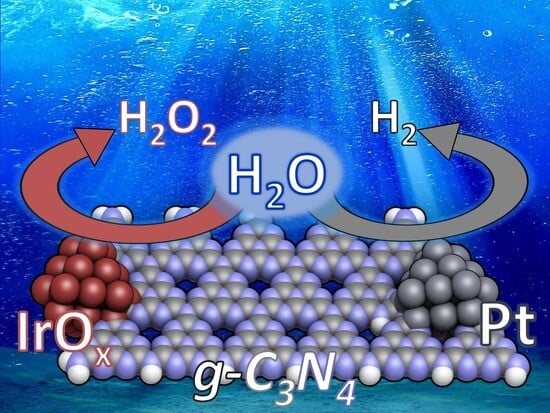
Bimetallic Pt-IrOx/g-C3N4 Photocatalysts for the Highly Efficient Overall Water Splitting under Visible Light
Abstract
:1. Introduction
2. Results
2.1. Preparation of the Pt-Ir/C3N4 Photocatalysts
2.2. Photocatalyst Characterization
2.3. Photocatalytic Tests
3. Materials and Methods
3.1. Starting Reagents and Physical Measurements
3.2. Preparation of Pt-Ir/g-C3N4 Catalysts
- Deposition of Pt on g-C3N4. To prepare catalysts with 0.5 or 0.1 wt.% of Pt, an appropriate aliquot (18.0 or 3.60 mL, respectively) of a (Me4N)2[Pt2(μ-OH)2(NO3)8] acetone solution (1.82 mM) was added to a suspension of g-C3N4 (2500 mg) in acetone (40 mL). The resulting suspension was stirred for 12 h at room temperature in a closed vial. The completion of sorption of platinum was checked by the absence of light absorption at 380 nm. The solid was collected by filtration, washed with a copious amount of acetone, and dried in an airflow at room temperature for 20 min.
- Calcination in H2. The resulting material was calcined in hydrogen (400 °C, 10 °C/min ramping, 1 h exposure time) to give a Pt(X)/g-C3N4 precursor. Here, X is the loading of Pt (0.5 or 0.1).
- Deposition of Ir. The precursor (500.0 mg) was dispersed in 6 mL of acetone, and then an appropriate aliquot (Table 4) of a fac-[Ir(H2O)3(NO2)3] solution ([Ir] = 4.00 mM) was added. The prepared suspension was thoroughly mixed using ultrasonication (10 min) and then dried in an airflow (200 °C) until complete removal of the solvent.
- Calcination in air. The obtained powder was calcined in an airflow at 350 °C for 1 h.
3.3. Apparatus
3.4. Photocatalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.; Huang, H. Precursor-Reforming Protocol to 3D Mesoporous g-C3N4 Established by Ultrathin Self-Doped Nanosheets for Superior Hydrogen Evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.-A.; Wang, X. Conjugated Polymers: Catalysts for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Lian, J.; Zhu, X.; Yan, P.; Lei, Y.; Yuan, S.; et al. Construction of MnO2/Monolayer g-C3N4 with Mn Vacancies for Z-Scheme Overall Water Splitting. Appl. Catal. B Environ. 2019, 241, 452–460. [Google Scholar] [CrossRef]
- Zheng, D.; Cao, X.-N.; Wang, X. Precise Formation of a Hollow Carbon Nitride Structure with a Janus Surface to Promote Water Splitting by Photoredox Catalysis. Angew. Chem. Int. Ed. 2016, 55, 11512–11516. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode One and Two-Dimensional Structure of Alpha-Helix and Beta-Sheet Forms of Poly (L-Alanine) Shown by Specific Heat Measurements at Low Temperatures (1.5–20 K). Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-Dimensional Porous g-C3N4 for Highly Efficient Photocatalytic Overall Water Splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Navakoteswara Rao, V.; Lakshmana Reddy, N.; Preethi, V.; Karthik, M.; Yu, Y.T.; Yang, J.M.; Mamatha Kumari, M.; Shankar, M.V. A Critical Review on Core/Shell-Based Nanostructured Photocatalysts for Improved Hydrogen Generation. Int. J. Hydrogen Energy 2023, 48, 11754–11774. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; He, H.; Yang, S.; Jia, G.; Liu, S. CoP Imbedded G-C3N4 Heterojunctions for Highly Efficient Photo, Electro and Photoelectrochemical Water Splitting. J. Colloid Interface Sci. 2021, 599, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, E.A.; Parmon, V.N. Heterogeneous Semiconductor Photocatalysts for Hydrogen Production from Aqueous Solutions of Electron Donors. Russ. Chem. Rev. 2017, 86, 870–906. [Google Scholar] [CrossRef]
- Bharagav, U.; Ramesh Reddy, N.; Nava Koteswara Rao, V.; Ravi, P.; Sathish, M.; Rangappa, D.; Prathap, K.; Shilpa Chakra, C.; Shankar, M.V.; Appels, L.; et al. Bifunctional G-C3N4/Carbon Nanotubes/WO3 Ternary Nanohybrids for Photocatalytic Energy and Environmental Applications. Chemosphere 2023, 311, 137030. [Google Scholar] [CrossRef]
- Hainer, A.S.; Hodgins, J.S.; Sandre, V.; Vallieres, M.; Lanterna, A.E.; Scaiano, J.C. Photocatalytic Hydrogen Generation Using Metal-Decorated TiO2: Sacrificial Donors vs. True Water Splitting. ACS Energy Lett. 2018, 3, 542–545. [Google Scholar] [CrossRef]
- Solakidou, M.; Giannakas, A.; Georgiou, Y.; Boukos, N.; Louloudi, M.; Deligiannakis, Y. Efficient Photocatalytic Water-Splitting Performance by Ternary CdS/Pt-N-TiO2 and CdS/Pt-N,F-TiO2: Interplay between CdS Photo Corrosion and TiO2-Dopping. Appl. Catal. B Environ. 2019, 254, 194–205. [Google Scholar] [CrossRef]
- He, H.; Cao, J.; Guo, M.; Lin, H.; Zhang, J.; Chen, Y.; Chen, S. Distinctive Ternary CdS/Ni2P/g-C3N4 Composite for Overall Water Splitting: Ni2P Accelerating Separation of Photocarriers. Appl. Catal. B Environ. 2019, 249, 246–256. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Chen, H.; Zhang, Y.; Zhang, F.; Liu, S.F. Fabrication of TiO2/C3N4 Heterostructure for Enhanced Photocatalytic Z-Scheme Overall Water Splitting. Appl. Catal. B Environ. 2016, 191, 130–137. [Google Scholar] [CrossRef]
- Yu, J.; Wu, S.; Zhao, X.; Li, Z.; Yang, X.; Shen, Q.; Lu, M.; Xie, X.; Zhan, D.; Yan, J. Progress on Two-Dimensional Transitional Metal Dichalcogenides Alloy Materials: Growth, Characterisation, and Optoelectronic Applications. Nanomaterials 2023, 13, 2843. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Si, Y.; Wang, M.; Liu, M.; Guo, L. Toward Efficient Photocatalytic Pure Water Splitting for Simultaneous H2 and H2O2 Production. Nano Energy 2019, 62, 823–831. [Google Scholar] [CrossRef]
- Wang, Z.; Inoue, Y.; Hisatomi, T.; Ishikawa, R.; Wang, Q.; Takata, T.; Chen, S.; Shibata, N.; Ikuhara, Y.; Domen, K. Overall Water Splitting by Ta3N5 Nanorod Single Crystals Grown on the Edges of KTaO3 Particles. Nat. Catal. 2018, 1, 756–763. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, C.; Wu, D.; Wang, X.; Wang, H.; Gao, J.; Huang, H.; Shi, C.; Liu, Y.; Kang, Z. Efficient Photocatalytic Water Splitting through Titanium Silicalite Stabilized CoO Nanodots. Nanoscale 2019, 11, 15984–15990. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H 2 -Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Hu, S.; Sun, X.; Zhao, Y.; Li, W.; Wang, H.; Wu, G. The Effective Photocatalytic Water Splitting to Simultaneously Produce H2 and H2O2 over Pt Loaded K-g-C3N4 Catalyst. J. Taiwan Inst. Chem. Eng. 2020, 107, 129–138. [Google Scholar] [CrossRef]
- Lu, W.; Yi, Z.; Zhang, J.; Xu, X.; Tang, B.; Li, G.; Zeng, L.; Chen, J.; Sun, T. A Tunable Broadband Absorber in the Terahertz Band Based on the Proportional Structure of a Single Layer of Graphene. Diam. Relat. Mater. 2023, 140, 110481. [Google Scholar] [CrossRef]
- Zhou, S.; Bi, K.; Li, Q.; Mei, L.; Niu, Y.; Fu, W.; Han, S.; Zhang, S.; Mu, J.; Tan, L.; et al. Patterned Graphene-Based Metamaterials for Terahertz Wave Absorption. Coatings 2023, 13, 59. [Google Scholar] [CrossRef]
- Lai, R.; Chen, H.; Zhou, Z.; Yi, Z.; Tang, B.; Chen, J.; Yi, Y.; Tang, C.; Zhang, J.; Sun, T. Design of a Penta-Band Graphene-Based Terahertz Metamaterial Absorber with Fine Sensing Performance. Micromachines 2023, 14, 1802. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Sun, X.; Hu, S.; Ma, H.; Wang, F.; Wu, G. Photocatalytic Water Splitting to Simultaneously Produce H2 and H2O2 by Two-Electron Reduction Process over Pt Loaded Na+ Introduced g-C3N4 Catalyst. Diam. Relat. Mater. 2020, 108, 107971. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wang, R.; Wu, M.-Z.; Yuan, Y.-P. A Review on G-C3N4 for Photocatalytic Water Splitting and CO2 Reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting via a Two-Electron Pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, C.; Wang, L.; Guo, S.; Zhang, Y.; Li, H.; Huang, H.; Liu, Y.; Tang, J.; Kang, Z. Control Strategy on Two-/Four-Electron Pathway of Water Splitting by Multidoped Carbon Based Catalysts. ACS Catal. 2017, 7, 1637–1645. [Google Scholar] [CrossRef]
- Zhurenok, A.V.; Vasilchenko, D.B.; Kozlova, E.A. Comprehensive Review on G-C3N4-Based Photocatalysts for the Photocatalytic Hydrogen Production under Visible Light. Int. J. Mol. Sci. 2023, 24, 346. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Zhurenok, A.; Saraev, A.; Gerasimov, E.; Cherepanova, S.; Tkachev, S.; Plusnin, P.; Kozlova, E. Highly Efficient Hydrogen Production under Visible Light over G-C3N4-Based Photocatalysts with Low Platinum Content. Chem. Eng. J. 2022, 445, 136721. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Tkachenko, P.; Tkachev, S.; Popovetskiy, P.; Komarov, V.; Asanova, T.; Asanov, I.; Filatov, E.; Maximovskiy, E.; Gerasimov, E.; et al. Sulfuric Acid Solutions of [Pt(OH)4(H2O)2]: A Platinum Speciation Survey and Hydrated Pt(IV) Oxide Formation for Practical Use. Inorg. Chem. 2022, 61, 9667–9684. [Google Scholar] [CrossRef] [PubMed]
- Topchiyan, P.; Vasilchenko, D.; Tkachev, S.; Sheven, D.; Eltsov, I.; Asanov, I.; Sidorenko, N.; Saraev, A.; Gerasimov, E.; Kurenkova, A.; et al. Highly Active Visible Light-Promoted Ir/g-C3N4 Photocatalysts for the Water Oxidation Reaction Prepared from a Halogen-Free Iridium Precursor. ACS Appl. Mater. Interfaces 2022, 14, 35600–35612. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, C. Photocatalytic Water Splitting on Semiconductor-Based Photocatalysts. Adv. Catal. 2017, 60, 1–57. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent Developments in Heterogeneous Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Maheskumar, V.; Saravanakumar, K.; Yea, Y.; Yoon, Y.; Park, C.M. Construction of Heterostructure Interface with FeNi2S4 and CoFe Nanowires as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting and Urea Electrolysis. Int. J. Hydrog. Energy 2023, 48, 5080–5094. [Google Scholar] [CrossRef]
- Maheskumar, V.; Saravanakumar, K.; Govindan, J.; Park, C.M. Rational Design of Double Perovskite La2Ni0.5Co0.5MnO6 Decorated Polyaniline Array on MoO3 Nanobelts with Strong Heterointerface Boosting Oxygen Evolution Reaction and Urea Oxidation. Appl. Surf. Sci. 2023, 612, 155737. [Google Scholar] [CrossRef]
- Pan, Z.; Zheng, Y.; Guo, F.; Niu, P.; Wang, X. Decorating CoP and Pt Nanoparticles on Graphitic Carbon Nitride Nanosheets to Promote Overall Water Splitting by Conjugated Polymers. ChemSusChem 2017, 10, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lan, Z.-A.; Lin, L.; Lin, S.; Wang, X. Overall Water Splitting by Pt/g-C3N4 Photocatalysts without Using Sacrificial Agents. Chem. Sci. 2016, 7, 3062–3066. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, S.; Niu, P.; Liu, M.; Wang, X. Photocatalytic Overall Water Splitting by Spatially-Separated Rh and RhOx Cocatalysts on Polymeric Carbon Nitride Nanosheets. J. Catal. 2019, 379, 129–137. [Google Scholar] [CrossRef]
- Mosiori, C.O.; Njoroge, W.K.; Ochoo, L.O. Optical Analysis of Ag-NPs Containing Methyl Ammonium Lead Tri-Iodide Thin Films. Path Sci. 2017, 3, 2007–2015. [Google Scholar] [CrossRef]
- Navakoteswara Rao, V.; Ravi, P.; Sathish, M.; Cheralathan, K.K.; Neppolian, B.; Mamatha Kumari, M.; Shankar, M.V. Manifestation of Enhanced and Durable Photocatalytic H2 Production Using Hierarchically Structured Pt@Co3O4/TiO2 Ternary Nanocomposite. Ceram. Int. 2021, 47, 10226–10235. [Google Scholar] [CrossRef]
- Moon, H.S.; Hsiao, K.; Wu, M.; Yun, Y.; Hsu, Y.; Yong, K. Spatial Separation of Cocatalysts on Z-Scheme Organic/Inorganic Heterostructure Hollow Spheres for Enhanced Photocatalytic H2 Evolution and In-Depth Analysis of the Charge-Transfer Mechanism. Adv. Mater. 2023, 35, 2200172. [Google Scholar] [CrossRef]
- Li, Z.; Kong, C.; Lu, G. Visible Photocatalytic Water Splitting and Photocatalytic Two-Electron Oxygen Formation over Cu- and Fe-Doped g-C3N4. J. Phys. Chem. C 2016, 120, 56–63. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Y.; Li, H.; Xu, Y.; Chen, X. 2D BiVO4/g-C3N4 Z-Scheme Photocatalyst for Enhanced Overall Water Splitting. J. Mater. Sci. 2019, 54, 10836–10845. [Google Scholar] [CrossRef]
- Dai, D.; Wang, P.; Bao, X.; Xu, Y.; Wang, Z.; Guo, Y.H.; Wang, Z.; Zheng, Z.; Liu, Y.; Cheng, H.; et al. G-C3N4/ITO/Co-BiVO4 Z-Scheme Composite for Solar Overall Water Splitting. Chem. Eng. J. 2022, 433, 134476. [Google Scholar] [CrossRef]
- Xu, B.B.; Fu, X.B.; You, X.M.; Zhao, E.; Li, F.F.; Chen, Z.; Li, Y.X.; Wang, X.L.; Yao, Y.F. Synergistic Promotion of Single-Atom Co Surrounding a PtCo Alloy Based on a g-C3N4 Nanosheet for Overall Water Splitting. ACS Catal. 2022, 12, 6958–6967. [Google Scholar] [CrossRef]
- Gong, Q.; Zhou, Y.; Wang, R.; Jiao, W. Enhanced Photocatalytic Pure Water Splitting of Porous G-C3N4/CdS Composite by the Bimetallic Phosphide. J. Environ. Chem. Eng. 2022, 10, 108046. [Google Scholar] [CrossRef]
- Xue, F.; Si, Y.; Cheng, C.; Fu, W.; Chen, X.; Shen, S.; Wang, L.; Liu, M. Electron Transfer via Homogeneous Phosphorus Bridges Enabling Boosted Photocatalytic Generation of H2 and H2O2 from Pure Water with Stoichiometric Ratio. Nano Energy 2022, 103, 107799. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wu, Q.; Wang, X.; Nie, H.; Zhou, Y.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Charge Storage of Carbon Dot Enhances Photo-Production of H2 and H2O2 over Ni2P/Carbon Dot Catalyst under Normal Pressure. Chem. Eng. J. 2021, 409, 128184. [Google Scholar] [CrossRef]
- Ai, Z.; Zhang, K.; Xu, L.; Huang, M.; Shi, D.; Shao, Y.; Shen, J.; Wu, Y.; Hao, X. In Situ Configuration of Dual S-Scheme BP/(Ti3C2Tx@TiO2) Heterojunction for Broadband Spectrum Solar-Driven Photocatalytic H2 Evolution in Pure Water. J. Colloid Interface Sci. 2022, 610, 13–23. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, W.; Zhang, Y.; Song, X.; Jiang, H. Precisely Anchoring Ni-Doped Cobalt Phosphide Nanoparticles on Phosphatized Carbon Nitride for Efficient Photocatalytic Water Splitting. Chem. Eng. J. 2023, 472, 144898. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, Y.; Cao, J.; Wang, H.; Wang, X.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. A 4e−–2e− Cascaded Pathway for Highly Efficient Production of H2 and H2O2 from Water Photo-Splitting at Normal Pressure. Appl. Catal. B Environ. 2020, 270, 118875. [Google Scholar] [CrossRef]
- Dou, Y.; Zhu, C.; Zhu, M.; Fu, Y.; Wang, H.; Shi, C.; Huang, H.; Liu, Y.; Kang, Z. Highly Mesoporous Carbon Nitride Photocatalysts for Efficient and Stable Overall Water Splitting. Appl. Surf. Sci. 2020, 509, 144706. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, Q.; Huang, J.; Liu, J.; Tan, G.; Sun, C.; Li, T.; Liu, S.; Li, Y.; Wang, H.; et al. Visually Resolving the Direct Z-Scheme Heterojunction in CdS@ZnIn2S4 Hollow Cubes for Photocatalytic Evolution of H2 and H2O2 from Pure Water. Appl. Catal. B Environ. 2021, 293, 120213. [Google Scholar] [CrossRef]
- Fang, W.L.; Wang, L.; Li, C.H. Preparation of Au-OVs-BiOBr-P25 Z-Scheme Photocatalyst and Its Photocatalytic Performance in Overall Water Splitting. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2022, 50, 446–455. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Zhao, Y.; Liu, Y.; Wu, Q.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Phosphorus-Doped Porous Carbon Nitride for Efficient Sole Production of Hydrogen Peroxide via Photocatalytic Water Splitting with a Two-Channel Pathway. J. Mater. Chem. A 2020, 8, 3701–3707. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Topchiyan, P.; Berdyugin, S.; Filatov, E.; Tkachev, S.; Baidina, I.; Komarov, V.; Slavinskaya, E.; Stadnichenko, A.; Gerasimov, E. Tetraalkylammonium Salts of Platinum Nitrato Complexes: Isolation, Structure, and Relevance to the Preparation of PtOx/CeO2 Catalysts for Low-Temperature CO Oxidation. Inorg. Chem 2019, 58, 6075–6087. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.A. TOPAS-Academic, Version 6 (Computer Software); Coelho Softwafe: Brisbane, Australia, 2007. [Google Scholar]
- Shirley, D.A. High-Resolution X-ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Bally, R.W.; Gribnau, T.C.J. Some Aspects of the Chromogen 3,3′,5,5’-Tetramethylbenzidine as Hydrogen Donor in a Horseradish Peroxidase Assay. Clin. Chem. Lab. Med. 1989, 27, 791–796. [Google Scholar] [CrossRef]






| Sample (Preparation Method) | SBET, m2·g−1 | V, cm3·g−1 |
|---|---|---|
| g-C3N4 | 47.5 | 0.24 |
| Pt0.5Ir0.1/C3N4(1) | 97.2 | 0.37 |
| Pt0.5Ir0.05/C3N4(1) | 99.3 | 0.28 |
| Pt0.5Ir0.01/C3N4(1) | 101 | 0.36 |
| Pt0.1Ir0.1/C3N4(1) | 76.8 | 0.41 |
| Pt0.1Ir0.5/C3N4(1) | 86.4 | 0.45 |
| Pt0.5Ir0.5/C3N4(2) | 120 | 0.40 |
| # | Sample | [Pt]/[C] | %, Pt0 | [Ir]/[C] | %, Ir0 | [Pt]/[Ir] | [Pt0]/[Ir3+] |
|---|---|---|---|---|---|---|---|
| Method 1 | |||||||
| 1 | Pt0.1Ir0.5/C3N4(1) | 0.0003 | 24 | 0.0015 | 14 | 0.2 | 0.06 |
| 2 | Pt0.1Ir0.1/C3N4(1) | 0.0004 | 39 | 0.0003 | 13 | 1.5 | 0.6 |
| 3 | Pt0.1Ir0.05/C3N4(1) | 0.0003 | 53 | 0.0002 | 33 | 1.8 | 1.7 |
| 4 | Pt0.1Ir0.01/C3N4(1) | 0.0003 | 60 | - | - | - | - |
| 5 | Pt0.5Ir0.5/C3N4(1) | 0.0021 | 50 | 0.0012 | 17 | 1.7 | 1.1 |
| 6 | Pt0.5Ir0.1/C3N4(1) | 0.0018 | 57 | 0.0002 | 0 | 10.5 | 5.9 |
| 7 | Pt0.5Ir0.05/C3N4(1) | 0.0018 | 68 | 0.0001 | 0 | 14.2 | 9.7 |
| 8 | Pt0.5Ir0.01/C3N4(1) | 0.0018 | 60 | - | - | - | - |
| Method 2 | |||||||
| 9 | Pt0.5Ir0.5/C3N4(2) | 0.0006 | 100 | 0.0005 | 100 | 1.23 | - |
| Method 3 | |||||||
| 10 | Pt0.5Ir0.5/C3N4(3) | 0.0011 | 84 | 0.0006 | 100 | 1.16 | - |
| # | Method | Metal Loading | Ultrapure H2O | 10% TEOA Solution | |||
|---|---|---|---|---|---|---|---|
| W(H2), μmol min−1 | Activity, μmol H2 h−1 gcat−1 | Activity, μmol H2O2 h−1 gcat−1 | W(H2), μmol min−1 | Activity, μmol H2 h−1 gcat−1 | |||
| 1 | 1 | Pt0.5/C3N4(1) * | - | - | - | 6.28 | 7540 |
| 2 | Pt0.5/C3N4(1) | - | - | - | 4.85 | 5820 | |
| 3 | Ir 0.5/C3N4(1) | - | - | - | - | - | |
| 4 | Pt0.5Ir0.5/C3N4(1) | 0.023 | 55.2 | 29.4 | 1.06 | 1270 | |
| 5 | Pt0.5Ir0.1/C3N4(1) | 0.023 | 55.2 | 44.1 | 0.46 | 552 | |
| 6 | Pt0.5Ir0.05/C3N4(1) | 0.026 | 62.4 | 62.7 | 0.36 | 432 | |
| 7 | Pt0.5Ir0.01/C3N4(1) | 0.043 | 103 | 16.8 | 0.46 | 552 | |
| 8 | Pt0.1Ir0.5/C3N4(1) | 0.016 | 38.4 | 27.3 | 0.13 | 156 | |
| 9 | Pt0.1Ir0.1/C3N4(1) | 0.019 | 45.6 | 35.7 | 0.06 | 72 | |
| 10 | Pt0.1Ir0.05/C3N4(1) | 0.026 | 62.4 | 39.9 | 0.37 | 444 | |
| 11 | Pt0.1Ir0.01/C3N4(1) | 0.036 | 86.4 | 71.4 | 0.45 | 540 | |
| 12 | Pt0.1/C3N4(1) | - | - | - | 2.79 | 3350 | |
| 13 | 2 | Pt0.5Ir0.5/C3N4(2) | - | - | - | 7.18 | 8620 |
| 14 | 3 | Pt0.5Ir0.5/C3N4(3) | - | - | - | 2.74 | 3290 |
| Ir wt.% in a Final Catalyst | 0.5 | 0.1 | 0.05 | 0.01 |
|---|---|---|---|---|
| VIr (μL) | 3266 | 653 | 327 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorenko, N.D.; Topchiyan, P.A.; Saraev, A.A.; Gerasimov, E.Y.; Zhurenok, A.V.; Vasilchenko, D.B.; Kozlova, E.A. Bimetallic Pt-IrOx/g-C3N4 Photocatalysts for the Highly Efficient Overall Water Splitting under Visible Light. Catalysts 2024, 14, 225. https://doi.org/10.3390/catal14040225
Sidorenko ND, Topchiyan PA, Saraev AA, Gerasimov EY, Zhurenok AV, Vasilchenko DB, Kozlova EA. Bimetallic Pt-IrOx/g-C3N4 Photocatalysts for the Highly Efficient Overall Water Splitting under Visible Light. Catalysts. 2024; 14(4):225. https://doi.org/10.3390/catal14040225
Chicago/Turabian StyleSidorenko, Nikolay D., Polina A. Topchiyan, Andrey A. Saraev, Evgeny Yu. Gerasimov, Angelina V. Zhurenok, Danila B. Vasilchenko, and Ekaterina A. Kozlova. 2024. "Bimetallic Pt-IrOx/g-C3N4 Photocatalysts for the Highly Efficient Overall Water Splitting under Visible Light" Catalysts 14, no. 4: 225. https://doi.org/10.3390/catal14040225
APA StyleSidorenko, N. D., Topchiyan, P. A., Saraev, A. A., Gerasimov, E. Y., Zhurenok, A. V., Vasilchenko, D. B., & Kozlova, E. A. (2024). Bimetallic Pt-IrOx/g-C3N4 Photocatalysts for the Highly Efficient Overall Water Splitting under Visible Light. Catalysts, 14(4), 225. https://doi.org/10.3390/catal14040225