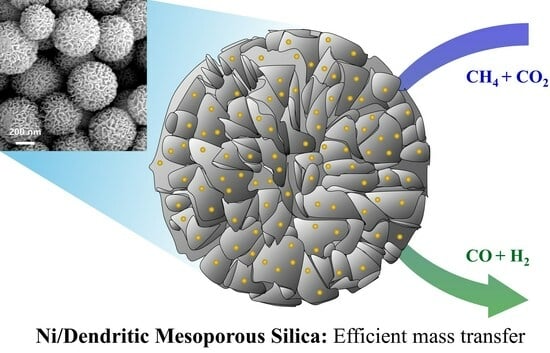
Enhanced Methane Dry Reforming with Ni/SiO2 Catalysts Featuring Hierarchical External Nanostructures
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Methodology
3.1. Catalyst Preparation
3.1.1. Synthesis of Dendritic Mesoporous Silica (DMS)
3.1.2. Synthesis of Spray EISA and Oven EISA Silica Supports
3.1.3. Synthesis of Ni/Silica Supported Catalysts
3.2. Characterization
3.3. Catalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biswas, S.; Kulkarni, A.P.; Giddey, S.; Bhattacharya, S. A Review on Synthesis of Methane as a Pathway for Renewable Energy Storage with a Focus on Solid Oxide Electrolytic Cell-Based Processes. Front. Energy Res. 2020, 8, 570112. [Google Scholar] [CrossRef]
- Al-Otaibi, F.; Xiao, H.; Berrouk, A.S.; Polychronopoulou, K. Numerical Study of Dry Reforming of Methane in Packed and Fluidized Beds: Effects of Key Operating Parameters. ChemEngineering 2023, 7, 57. [Google Scholar] [CrossRef]
- Chein, R.; Yang, Z. Experimental Study on Dry Reforming of Biogas for Syngas Production over Ni-Based Catalysts. ACS Omega 2019, 4, 20911–20922. [Google Scholar] [CrossRef] [PubMed]
- Ingale, P.; Guan, C.; Kraehnert, R.; Naumann d’Alnoncourt, R.; Thomas, A.; Rosowski, F. Design of an Active and Stable Catalyst for Dry Reforming of Methane via Molecular Layer Deposition. Catal. Today 2021, 362, 47–54. [Google Scholar] [CrossRef]
- Torimoto, M.; Sekine, Y. Effects of Alloying for Steam or Dry Reforming of Methane: A Review of Recent Studies. Catal. Sci. Technol. 2022, 12, 3387–3411. [Google Scholar] [CrossRef]
- Le Saché, E.; Reina, T.R. Analysis of Dry Reforming as Direct Route for Gas Phase CO2 Conversion. The Past, the Present and Future of Catalytic DRM Technologies. Prog. Energy Combust. Sci. 2022, 89, 100970. [Google Scholar] [CrossRef]
- Han, K.; Yu, W.; Xu, L.; Deng, Z.; Yu, H.; Wang, F. Reducing Carbon Deposition and Enhancing Reaction Stability by Ceria for Methane Dry Reforming over Ni@SiO2@CeO2 Catalyst. Fuel 2021, 291, 120182. [Google Scholar] [CrossRef]
- Song, Q.; Ran, R.; Wu, X.; Si, Z.; Weng, D. Dry Reforming of Methane over Ni Catalysts Supported on Micro- and Mesoporous Silica. J. CO2 Util. 2023, 68, 102387. [Google Scholar] [CrossRef]
- Klaewkla, R.; Arend, M.; Hoelderich, W.F. A Review of Mass Transfer Controlling the Reaction Rate in Heterogeneous Catalytic Systems. In Mass Transfer—Advanced Aspects; Nakajima, H., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-636-2. [Google Scholar]
- Zhang, Y.; Zhang, G.; Liu, J.; Li, T.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, Y. Dry Reforming of Methane over Ni/SiO2 Catalysts: Role of Support Structure Properties. Fuel 2023, 340, 127490. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Chen, C.; Jia, L.; Hou, B.; Li, D. Effects of Macropores on Reducing Internal Diffusion Limitations in Fischer–Tropsch Synthesis Using a Hierarchical Cobalt Catalyst. RSC Adv. 2017, 7, 9436–9445. [Google Scholar] [CrossRef]
- Wang, G.; Coppens, M.-O. Rational Design of Hierarchically Structured Porous Catalysts for Autothermal Reforming of Methane. Chem. Eng. Sci. 2010, 65, 2344–2351. [Google Scholar] [CrossRef]
- Al-Halhouli, M.; Kieninger, J.; Yurchenko, O.; Urban, G. Mass Transport and Catalytic Activity in Hierarchical/Non-Hierarchical and Internal/External Nanostructures: A Novel Comparison Using 3D Simulation. Appl. Catal. Gen. 2016, 517, 12–20. [Google Scholar] [CrossRef]
- Shen, D.; Chen, L.; Yang, J.; Zhang, R.; Wei, Y.; Li, X.; Li, W.; Sun, Z.; Zhu, H.; Abdullah, A.M.; et al. Ultradispersed Palladium Nanoparticles in Three-Dimensional Dendritic Mesoporous Silica Nanospheres: Toward Active and Stable Heterogeneous Catalysts. ACS Appl. Mater. Interfaces 2015, 7, 17450–17459. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.-L.; Lang, W.-Z.; Yan, X.; Guo, Y.-J. Dispersed Vanadium in Three-Dimensional Dendritic Mesoporous Silica Nanospheres: Active and Stable Catalysts for the Oxidative Dehydrogenation of Propane in the Presence of CO2. ACS Appl. Mater. Interfaces 2017, 9, 15408–15423. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Jiang, P.; Shen, Y.; Zhang, K.; Wai, P.T.; Haryono, A. High-Dispersed MoO3 Nanoparticles in 3D-Dendritic Mesoporous Silica Nanospheres: Heterogeneous Catalysts for the Epoxidation of Olefins. J. Porous Mater. 2021, 28, 779–789. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, X.; Han, X.; You, X.; Lin, S.; Chen, H.; Liu, W.; Wang, X.; Zhang, N.; Wang, Z.; et al. Catalysts in Coronas: A Surface Spatial Confinement Strategy for High-Performance Catalysts in Methane Dry Reforming. ACS Catal. 2019, 9, 9072–9080. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Synergetic Effect of Yolk–Shell Structure and Uniform Mixing of SnS–MoS2 Nanocrystals for Improved Na-Ion Storage Capabilities. ACS Appl. Mater. Interfaces 2015, 7, 24694–24702. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A Review on Dry Reforming of Methane in Aspect of Catalytic Properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Molina, R.; Poncelet, G. α-Alumina-Supported Nickel Catalysts Prepared from Nickel Acetylacetonate: A TPR Study. J. Catal. 1998, 173, 257–267. [Google Scholar] [CrossRef]
- Xie, T.; Shi, L.; Zhang, J.; Zhang, D. Immobilizing Ni Nanoparticles to Mesoporous Silica with Size and Location Control via a Polyol-Assisted Route for Coking- and Sintering-Resistant Dry Reforming of Methane. Chem. Commun. 2014, 50, 7250–7253. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Li, J.; Wei, S.; Gao, X.; Wang, P. Combustion-Impregnation Preparation of Ni/SiO2 Catalyst with Improved Low-Temperature Activity for CO2 Methanation. Int. J. Hydrog. Energy 2021, 46, 20919–20929. [Google Scholar] [CrossRef]
- Singha, R.K.; Yadav, A.; Agrawal, A.; Shukla, A.; Adak, S.; Sasaki, T.; Bal, R. Synthesis of Highly Coke Resistant Ni Nanoparticles Supported MgO/ZnO Catalyst for Reforming of Methane with Carbon Dioxide. Appl. Catal. B Environ. 2016, 191, 165–178. [Google Scholar] [CrossRef]
- Huang, X.; Ni, C.; Zhao, G.; Irvine, J.T.S. Oxygen Storage Capacity and Thermal Stability of the CuMnO2–CeO2 Composite System. J. Mater. Chem. A 2015, 3, 12958–12964. [Google Scholar] [CrossRef]
- Aranda-Aguirre, A.; Ojeda, J.; Ferreira De Brito, J.; Garcia-Segura, S.; Boldrin Zanoni, M.V.; Alarcon, H. Photoelectrodes of Cu2O with Interfacial Structure of Topological Insulator Bi2Se3 Contributes to Selective Photoelectrocatalytic Reduction of CO2 towards Methanol. J. CO2 Util. 2020, 39, 101154. [Google Scholar] [CrossRef]
- Ji, K.; Meng, F.; Xun, J.; Liu, P.; Zhang, K.; Li, Z.; Gao, J. Carbon Deposition Behavior of Ni Catalyst Prepared by Combustion Method in Slurry Methanation Reaction. Catalysts 2019, 9, 570. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Park, E.D. CO and CO2 Methanation over Supported Cobalt Catalysts. Top. Catal. 2017, 60, 714–720. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Peng, H.; You, X.; Peng, C.; Xu, X.; Liu, W.; Fang, X.; Wang, Z.; Zhang, N.; et al. Nickel Nanoparticles Embedded in Mesopores of AlSBA-15 with a Perfect Peasecod-like Structure: A Catalyst with Superior Sintering Resistance and Hydrothermal Stability for Methane Dry Reforming. Appl. Catal. B Environ. 2018, 224, 488–499. [Google Scholar] [CrossRef]









| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Mean Pore Size (nm) |
|---|---|---|---|
| Ni/DMS | 450.0 | 1.24 | 11.0 |
| Ni/spray EISA | 304.7 | 0.69 | 9.0 |
| Ni/oven EISA | 709.0 | 0.74 | 4.2 |
| Catalysts | XRD | H2-Pulse Chemisorption | ||
|---|---|---|---|---|
| Ni Crystalline Size (nm) | Metallic Ni Surface Area (m2/gcat) | Ni Dispersion (%) | Average Ni Particle Diameter (nm) | |
| Ni/DMS | 9.4 | 74.3 | 11.1 | 9.0 |
| Ni/spray EISA | 10.3 | 52.6 | 7.9 | 12.8 |
| Ni/oven EISA | 11.6 | 65.9 | 9.9 | 10.2 |
| Samples | H2 Consumption (mmol/gcat) | First Peak Area (%) | Second Peak Area (%) |
|---|---|---|---|
| Ni/DMS | 0.09 | 34% | 63% |
| Ni/spray EISA | 0.09 | 46% | 54% |
| Ni/oven EISA | 0.07 | 52% | 48% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Kim, M.-J.; Kim, D.H.; Mnoyan, A.; Lee, K. Enhanced Methane Dry Reforming with Ni/SiO2 Catalysts Featuring Hierarchical External Nanostructures. Catalysts 2024, 14, 265. https://doi.org/10.3390/catal14040265
Kim YJ, Kim M-J, Kim DH, Mnoyan A, Lee K. Enhanced Methane Dry Reforming with Ni/SiO2 Catalysts Featuring Hierarchical External Nanostructures. Catalysts. 2024; 14(4):265. https://doi.org/10.3390/catal14040265
Chicago/Turabian StyleKim, Yong Jun, Min-Jae Kim, Dong Hyun Kim, Anush Mnoyan, and Kyubock Lee. 2024. "Enhanced Methane Dry Reforming with Ni/SiO2 Catalysts Featuring Hierarchical External Nanostructures" Catalysts 14, no. 4: 265. https://doi.org/10.3390/catal14040265
APA StyleKim, Y. J., Kim, M. -J., Kim, D. H., Mnoyan, A., & Lee, K. (2024). Enhanced Methane Dry Reforming with Ni/SiO2 Catalysts Featuring Hierarchical External Nanostructures. Catalysts, 14(4), 265. https://doi.org/10.3390/catal14040265