An Overview of Environmental Catalysis Mediated by Hydrogen Peroxide
Abstract
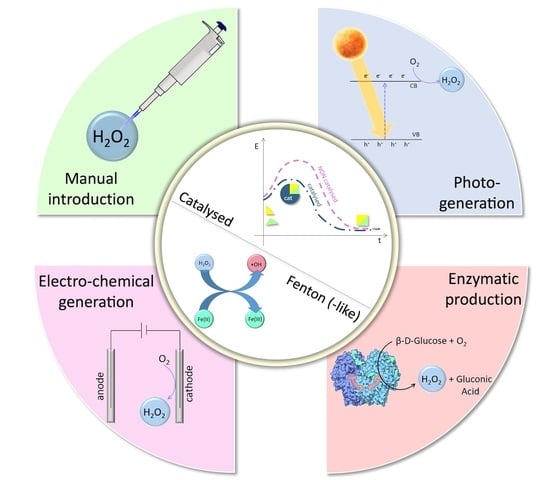
:1. Introduction
2. Recent Developments in AOP Systems Involving H2O2
2.1. Advanced Materials
2.1.1. Considerations on Structures and Morphologies
2.1.2. Considerations on Chemical Compositions and Stability
2.2. Enzyme-Driven Processes
2.2.1. Glucose-Oxidase-Based Bio-Fenton
| Pollutant | Performances | Conditions | Notes | Ref |
|---|---|---|---|---|
| 1.Trichloroethene (TCE) | Removal after 192 h - TCE 5 mg L−1: 76.2% - TCE 50 mg L−1: 94.1%. | 200 mg magnetic nanoparticles (MIG); 2.5 mM Glucose. | - Gox immobilized on MIG - Recycled for 4 cycles - Effectiveness in the ranges of T 15–45 °C and of pH 3.6–9.0 - Influence of inorganic ions: Ca2+ >Mg2 + >Cu2+ and H2PO4−> Cl−> SO42−. | [196] |
| 2. 4-chlorophenol (4-CP) | Removal after 250 min - GOx@Kaolin/OGR: 77.5% - GOx@Kaolin/OGR/UV: 96.1%. | 4-CP: 5 mg L−1 UV: 150 μW cm−2 T: 25 ± 1 °C OGR: 250 mg L−1 GOx@Kaolin: 2.5 U mL−1 Glucose: 5 mmol L−1. | - GOx immobilized on Kaolin (GOx@Kaolin) coupled with organic green rust (OGR, source of iron) and enhanced by UV light - Reusability 6 cycles. | [197] |
| 3.Trichloroethylene (TCE) | - After 24 h at pH 7 Ground water: 30% or 48% if doped with additional H2O2 (after 3 h). | TCE: 60 mg L−1 Glucose: 60 mM GOx: 1 mg mL−1 Fe: 25 mg L−1. | - GOx in solution - Organic matter of ground water acts as a radical scavenger. | [201] |
| 4. Polycyclic aromatic hydrocarbons (PAHs): Naphthalene (NAP), Anthracene (ANT), Pyrene (PYR) | - After 48 h, neutral pH, removal%: 95.1%, 75.4%, and 85.2% for NAP, ANT, and PYR - COD reduction%: 28.6%, 13.8%, and 30.8% for NAP, ANT, and PYR. | PAH: 50 mg L−1 each GOx: 10 U Fe(III)citrate: 0.1 mM Glucose: 2 mM. | - GOx in solution - Evaluation of PAHs concentration and COD to evaluate the mineralization - Bio-Fenton as pre-treatment to enhance PAHs removal by activated sludge: COD removal from 33% to 72%. | [189] |
| 5. Bisphenol A (BPA) | After 10 days of incubation Removal of 80%. | BPA: 0.1 mM GOx: 10 U Glucose: 32 mM Fe(III)citrate: 0.5 mM pH 5.3. | - GOx in solution. | [188] |
| 6. Chloro-acetanilide herbicides: acetochlor, alachlor, metolachlor, propachlor, butachlor | After 5 days, degradation %: acetochlor: 72.8% alachlor: 73.4% metolachlor: 74.0% propachlor: 47.4% butachlor: 43.8%. | GOx: 10 U pH 5.5 Fe(III)citrate: 0.5 mM Glucose: 32 mM Each herbicide 0.1 mM. | - GOx in solution - Influence of chemical structure of herbicides and particularly of R-O-R’ groups. | [187] |
| 7. Trace organic contaminant (TrOCs) (mefenamic acid, ketoprofen, caffeine, carbamazepine, trimethoprim, fenofibrate, diuron, carbendazim, thiabendazole) | After 360 min pH 7 mefenamic acid: 68.54% ketoprofene: 44.7% caffeine: 36.1% carbamazepine: 44.1% trimethoprim: 46.4% fenofibrate: 20.3% diuron: 89.4% carbendazim: 73.1% thiabendazole: 88.9%. | Glucose: 1 M GOx: 100 U mL−1 pH 7 50 µg L−1 mix of TrOCs T: 30 °C H2O2:FeSO4 = 50:1. | - GOx produced from Aspergillus niger using Casuarina equisetifolia biomass in a pilot-scale - Municipal wastewater as a matrix - Influence of the rate H2O2: FeSO4. | [185] |
| 8. Trichloroethylene (TCE) | After 8 h Removal of 78%. | Glucose: 2.5 mM Fe(II): 0.5 mM GOx: 10 U mL−1. | - GOx in solution - Efficiency maintained in the pH range 3–6 and in a T range 15–30 °C. | [184] |
| 9. Sulfonated polyethylene (SPE) | After 6 h with free GOx, concentration degradation products: Acetic acid: 0.22 mM Butanoic acid: 0.01 mM; After 6 h with TiO2-GOx: degradation product conc.: Acetic acid 4.78 mM Butanoic acid 0.17 mM. | GOx free: Glucose: 32 mM GOx: 1 U mL−1 pH 5.5, T: 30 °C SPE: 1 mg mL−1; TiO2-GOx: As above, except for 10 U of TiO2-GOx Xe lamp 150 W, 400 nm cut off. | - GOx both free and immobilized on TiO2 particles - Degradation follows studying the product formation (acetic acid, butanoic acid, isovaleric acid, 1,2-ethanediol monoacetate). | [200] |
| 10. Atrazine (ATZ) | After 360 min 72.8% removal. | Phosphate buffer: 5 mM GOx: 10 μmol min−1 Glucose: 3 mM Ferric citrate: 0.5 mM ATZ: 0.1 mM pH 5.8. | - GOx in solution - Toxicological assay and by-products study. | [190] |
| 11. 3, 4-Dimethylaniline (3, 4 DMA) | After 180 min, removal of 86.55%. | 3, 4-DMA: 30 mg L−1 Green Rush: 1 mM in Fe(II) and 1 mM in Fe(III) Glucose: 5 mM pH 7 Kaolin@GOx: 2.5 U mL−1. | - GOx immobilized on Kaolin (Kaolin@GOx) - Organic green rust as a source of iron. | [183] |
2.2.2. Bi-Enzymatic Processes for Water Treatment
Enzymatic Cycles Activated by H2O2
Glucose Oxidase Coupling with Peroxidases
3. Detection Methods for H2O2
| System | Wavelength | Conditions | Ref |
|---|---|---|---|
| 1. Phenolphthalein method | 552 nm | - 0.24 mL of phenolphthalein stock solution: 0.02 g mL−1 Phenolphthalein solution containing 10 g of NaOH, 5 g of zinc - CuSO4 solution: 0.48 mL, 0.01 M - 100 mL of water. | [234] |
| 2. Iodide method | 352 nm | -2 mL of solution A (66 g L−1 KI; 0.2 g L−1 of (NH4)6Mo7O24; 2 g L−1 of NaOH) - 2 mL of solution B (20 g L−1 of potassium hydrogen phthalate) - 6 mL of Peroxide samples. | [57,133,234,235] |
| 3. Oxidation of NADPH | 340 nm | - Phosphate buffer: 0.04 M, pH 7.75 - EDTA: 4 × 10−4 M - Sodium azide: 4.2 × 10−3 M - Glutathione peroxidase: 8 × 10−8 M - Reduced glutathione: 3 × 10−3 M - NADPH: 5.6 × 10−5 M - Glutathione reductase: 1 U -Peroxide sample. | [234] |
| 4. DMAB/MBTH/HRP | 590 nm | - 3-(dimethylamino)benzoic acid (DMAB): 5 × 10−4 M - 3-methyl-2-benzothiazolinonehydrazone (MBTH): 2 × 10−5 M - Acetate buffer: 0.1 M, pH 5.5 -HRP. | [280] |
| 5. 4-aminoantypirine/phenol/HRP | 505 nm | - 4 mL of 4-aminoantipyrine/phenol reagent (2.34 g L−1 of phenol, 1g L−1 of 4-aminoantipyrine, 0.001 M phosphate buffer pH 6.9, 2.5 µM HRP) - 6 mL of peroxide sample. | [234,236,237] |
| 6. Nanoparticles decorated Ce2(WO4)3 nanosheets (CWNSs) | 652 nm | - TMB: 100 µL 8 mM - CWNSs: 70 µL, 1000 µg mL−1 - Phosphate buffer: 400 µL, 50 mM pH 4 - MilliQ water: 330 µL - H2O2: 100 µL - LOD: 0.15 µM. | [281] |
| 7. Ammonium metavanadate | 450 nm | - Metavanadate 6.2 mM - Sulfuric acid 0.058 M - LOD: 143 µM. | [238,239,240] |
| 8. Fe3O4 magnetic nanoparticles (MNPs) with peroxidase mimetics | 545 nm | - ABTS: 24 µL, 60 mM - Fe3O4 MNPs: 10 µL, 3.74 mg mL−1 - Acetate buffer: pH 4185 µL - H2O2: 24 µL - Incubation 45 °C for 10 min and then diluted with 900 µL of water (after MNPs removal) and analyzed. | [282] |
| 9. Peroxidase-mimicking metal−organic framework containing catalytic Cu2+ and luminescent Tb3+: PA-Tb-Cu MOF (PA = m-phthalic acid) | 545 nm Fluorescence (310 nm exciting wavelength) | - Acetate buffer: 960 μL, 10 mM, pH 5.05 - 20 μL of PA-Tb-Cu - MOF suspension: 11.73 mg mL−1 - Ascorbic acid: 10 μL, 200 mM - H2O2 sample + water up to a final volume of 1 mL - Measure after 20 min incubation - LOD: 0.2 µM. | [244] |
| 10. CeO2 nanoparticles doped with Eu3+ | 590 nm Fluorescence (330 nm exciting wavelength) | - Samples prepared in potassium phosphate buffer (KPi) or Phosphate Buffered Saline (PBS) or 10% Farmigene Stain Buffer (FBS) - 125 g L−1 of nanoparticles - Measure after 30 min of incubation - LOD: 150 nM. | [243] |
| 11. Cobalt/bicarbonate system | 260 nm | - Sodium oxalate: 25 μL,16.34 mM - Cobalt chloride: 25 μL, 67.8 mM - 2 mL of sample Finally, the reaction volume is made up to 2.5 mL with 270 μL of saturated sodium bicarbonate solution. | [185,201,283,284] |
| 12. Titanium oxalate | 385 nm | - 10 mL of peroxide sample - 1 mL of 1 M sulfuric acid - 1 mL of 50 g L−1 potassium titanium oxalate solution - 13 mL of water - Measure after 5 min of incubation - LOD: 29 µM. | [77,125,285] |
| 13. N,N-diethyl-p-phenylenediamine (DPD) | 551 nm | - DPD reagent (27 mL water, 3 mL phosphate buffer, 6 µL methanol, 50 µL of 10 g L−1 of DPD solution prepared in sulfuric acid 0.5 M, 50 µL HRP 1g L−1) - LOD: 0.77 µM. | [64,285,286] |
| 14. p-hydroxyphenyl acetic acid (POHPAA) | 406–410 nm Fluorescence (315 nm exciting wavelength) | - POHPAA reagent (POHPAA 270 mg L−1, HRP 30 mg L−1, NaOH 1 M, potassium hydrogen phthalate 8.2 g L−1 pH 5.8) - LOD: 0.16 µM. | [285,287,288] |
4. Criticalities and Perspectives
4.1. Product Characterization, Toxicity and Influence of Environmental Factors
4.2. Processes’ Scalability, Cost Analysis and Environmental Impact
4.3. Final Considerations
- The use of AOPs in mild pH conditions can reduce additional process costs related to the salinity increase, as it is induced in classic Fenton treatment by acidification and further neutralization [315];
- In situ hydrogen peroxide production avoids high costs and hazards associated with its transport, handling, and storage;
- Homogeneous processes such as ozonization have been investigated and already applied in the full-scale treatment of urban wastewater effluents [327];
- Heterogeneous catalysis shows several advantages with respect to homogeneous processes, such as the recyclability of the catalyst, which can contribute both to cost reduction and limitation of the environmental impact. However, this strategy does not seem completely ready for full-scale application due to catalyst preparation costs, effectiveness, stability, and reactor configurations [328];
- The main hotspot of the environmental impact is energy and, consequently, an appropriate choice of energy source can significantly reduce the total impact of the process; thus, investment in renewable energies should be preferred;
- Since wastewaters are characterized by a complex composition, their contamination cannot be solved by employing a single remediation technique. Effective hybrid systems combining AOP techniques and/or biological treatments are reported in the literature [315];
- AOP strategies have been reported among the best available technologies in a recent review about wastewater reuse in European countries [315];
- Limited scale-up and techno-economic analysis are available for Bio-Fenton treatments. Enzyme cost and stability could represent critical points for its real applications; however, some studies suggest that GOx production could be cost-effective and scalable [185]. Moreover, as widely described above, the immobilization of glucose oxidase improves its stability towards oxidation and deactivation due to environmental factors and ensures its reusability, making Bio-Fenton suitable for actual wastewater treatment;
- Although, to the best of the authors’ knowledge, there are no reported pilot or full-scale plants that integrate Bio-Fenton in wastewater treatments, its effectiveness as a pretreatment to be coupled with conventional active sludge was highlighted by Wang et al. [189].
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.F. Water. In Sustainable Design and Build; Butterworth-Heinemann: Oxford, UK, 2019; pp. 301–418. ISBN 9780128167229. [Google Scholar]
- Velasco-Muñoz, J.; Aznar-Sánchez, J.; Belmonte-Ureña, L.; Román-Sánchez, I. Sustainable Water Use in Agriculture: A Review of Worldwide Research. Sustainability 2018, 10, 1084. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced Oxidation Processes for Water and Wastewater Viral Disinfection. A Systematic Review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef]
- Testa, M.L.; Tummino, M.L. Lignocellulose Biomass as a Multifunctional Tool for Sustainable Catalysis and Chemicals: An Overview. Catalysts 2021, 11, 125. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Siddique, M.S.; Yu, W. A Critical Review of Recent Progress in Global Water Reuse during 2019–2021 and Perspectives to Overcome Future Water Crisis. Environments 2023, 10, 159. [Google Scholar] [CrossRef]
- Roy, A.; Sharma, A.; Yadav, S.; Jule, L.T.; Krishnaraj, R. Nanomaterials for Remediation of Environmental Pollutants. Bioinorg. Chem. Appl. 2021, 2021, 1764647. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Arifin, M.N.; Jusoh, R.; Abdullah, H.; Ainirazali, N.; Setiabudi, H.D. Recent Advances in Advanced Oxidation Processes (AOPs) for the Treatment of Nitro- and Alkyl-Phenolic Compounds. Environ. Res. 2023, 229, 115936. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. ADVANCED OXIDATION PROCESS: A Remediation Technique for Organic and Non-Biodegradable Pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Casas, J.A. Intensification Strategies for Thermal H2O2-Based Advanced Oxidation Processes: Current Trends and Future Perspectives. Chem. Eng. J. Adv. 2022, 9, 100228. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; He, S.; Wu, S.; Yang, C. Singlet Oxygen: Properties, Generation, Detection, and Environmental Applications. J. Hazard. Mater. 2024, 461, 132538. [Google Scholar] [CrossRef] [PubMed]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of Catalytic Ozonation: An Investigation into Superoxide Ion Radical and Hydrogen Peroxide Formation during Catalytic Ozonation on Alumina and Zeolites in Water. Appl. Catal. B Environ. 2013, 129, 437–449. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Wang, H.; Lian, J.; Qiang, Z. Removal of Recalcitrant Organics in Reverse Osmosis Concentrate from Coal Chemical Industry by UV/H2O2 and UV/PDS: Efficiency and Kinetic Modeling. Chemosphere 2022, 287, 131999. [Google Scholar] [CrossRef] [PubMed]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- An, J.; Li, N.; Wu, Y.; Wang, S.; Liao, C.; Zhao, Q.; Zhou, L.; Li, T.; Wang, X.; Feng, Y. Revealing Decay Mechanisms of H2O2-Based Electrochemical Advanced Oxidation Processes after Long-Term Operation for Phenol Degradation. Environ. Sci. Technol. 2020, 54, 10916–10925. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jing, J.; Zhou, M.; Dewil, R. Recent Advances in H2O2-Based Advanced Oxidation Processes for Removal of Antibiotics from Wastewater. Chin. Chem. Lett. 2023, 34, 107621. [Google Scholar] [CrossRef]
- Tummino, M.L. SrFeO3 Peculiarities and Exploitation in Decontamination Processes and Environmentally-Friendly Energy Applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100339. [Google Scholar] [CrossRef]
- Qu, C.; Liang, D.W. Novel Electrochemical Advanced Oxidation Processes with H2O2 Generation Cathode for Water Treatment: A Review. J. Environ. Chem. Eng. 2022, 10, 107896. [Google Scholar] [CrossRef]
- Pi, L.; Cai, J.; Xiong, L.; Cui, J.; Hua, H.; Tang, D.; Mao, X. Generation of H2O2 by On-Site Activation of Molecular Dioxygen for Environmental Remediation Applications: A Review. Chem. Eng. J. 2020, 389, 123420. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhang, Y.; Yu, H.; Qu, Y.; Yu, J. Inorganic Metal-Oxide Photocatalyst for H2O2 Production. Small 2022, 18, 2104561. [Google Scholar] [CrossRef]
- Mansour, M.S.; Farid, Y.; Nosier, S.A.; Adli, O.; Abdel-Aziz, M.H. Removal of Eosin Yellow Dye from Industrial Wastewater Using UV/H2O2 and Photoelectro-Fenton Techniques. J. Photochem. Photobiol. A Chem. 2023, 436, 114411. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Rauf, M.; Luo, H.; Sun, X.; Jiang, Y. Gas Diffusion Electrodes for H2O2 Production and Their Applications for Electrochemical Degradation of Organic Pollutants in Water: A Review. Sci. Total Environ. 2021, 759, 143459. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.P.S.; Jafari, S.; Sillanpää, M.; Lee, G.J.; Wu, J.J.; Swaminathan, M. Recent Developments in Homogeneous Advanced Oxidation Processes for Water and Wastewater Treatment. Int. J. Photoenergy 2014, 2014, 821674. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced Oxidation Processes for In-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Textile Wastewater Treatment: A Review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Ji, J.; Wang, Z.; Xu, Q.; Zhu, Q.; Xing, M. In Situ H2O2 Generation and Corresponding Pollutant Removal Applications: A Review. Chem.—A Eur. J. 2023, 29, e202203921. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like Processes with in-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Degradation of Emerging Contaminants: Advances and Prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef]
- Popat, A.; Nidheesh, P.V.; Anantha Singh, T.S.; Suresh Kumar, M. Mixed Industrial Wastewater Treatment by Combined Electrochemical Advanced Oxidation and Biological Processes. Chemosphere 2019, 237, 124419. [Google Scholar] [CrossRef]
- Rezaei, F.; Vione, D. Effect of PH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef]
- Kamali, M.; Aminabhavi, T.M.; Costa, M.E.V.; Ul Islam, S.; Appels, L.; Dewil, R. Heterogeneous Advanced Oxidation Processes (HE-AOPs) for the Removal of Pharmaceutically Active Compounds—Pros and Cons. In Advanced Wastewater Treatment Technologies for the Removal of Pharmaceutically Active Compounds. Green Energy and Technology; Springer: Cham, Switzerland, 2023; pp. 211–239. [Google Scholar]
- Rodríguez-Chueca, J.; Carbajo, J.; García-Muñoz, P. Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review. Catalysts 2023, 13, 401. [Google Scholar] [CrossRef]
- Mazumder, A.; Bhattacharya, S.; Bhattacharjee, C. Role of Nano-Photocatalysis in Heavy Metal Detoxification. In Nanophotocatalysis and Environmental Applications; Springer Nature Switzerland: Cham, Switzerland, 2020; pp. 1–33. [Google Scholar]
- Tseng, D.H.; Juang, L.C.; Huang, H.H. Effect of Oxygen and Hydrogen Peroxide on the Photocatalytic Degradation of Monochlorobenzene in TiO2 Aqueous Suspension. Int. J. Photoenergy 2012, 2012, 328526. [Google Scholar] [CrossRef]
- Huang, X.; Song, M.; Zhang, J.; Shen, T.; Luo, G.; Wang, D. Recent Advances of Electrocatalyst and Cell Design for Hydrogen Peroxide Production. Nano-Micro Lett. 2023, 15, 86. [Google Scholar] [CrossRef]
- Kim, D.J.; Zhu, Q.; Rigby, K.; Wu, X.; Kim, J.H.; Kim, J.-H. A Protocol for Electrocatalyst Stability Evaluation: H2O2 Electrosynthesis for Industrial Wastewater Treatment. Environ. Sci. Technol. 2022, 56, 1365–1375. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Gao, J.; Alshawabkeh, A.N. Hydrogen Peroxide Generation from O2 Electroreduction for Environmental Remediation: A State-of-the-Art Review. Chemosphere 2019, 225, 588–607. [Google Scholar] [CrossRef]
- Knowles, R.R. Reaching Your Full (Over)Potential: A Novel Approach to Electrocatalytic Oxygen Reduction. ACS Cent. Sci. 2015, 1, 224–225. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, D.; Chu, C.; Mao, S. Photocatalytic H2O2 Production Systems: Design Strategies and Environmental Applications. Chem. Eng. J. 2023, 451, 138489. [Google Scholar] [CrossRef]
- An, J.; Feng, Y.; Zhao, Q.; Wang, X.; Liu, J.; Li, N. Electrosynthesis of H2O2 through a Two-Electron Oxygen Reduction Reaction by Carbon Based Catalysts: From Mechanism, Catalyst Design to Electrode Fabrication. Environ. Sci. Ecotechnol. 2022, 11, 100170. [Google Scholar] [CrossRef]
- Qian, W.; Wu, Z.; Jia, Y.; Hong, Y.; Xu, X.; You, H.; Zheng, Y.; Xia, Y. Thermo-Electrochemical Coupling for Room Temperature Thermocatalysis in Pyroelectric ZnO Nanorods. Electrochem. Commun. 2017, 81, 124–127. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of Iron-Based Materials in Heterogeneous Advanced Oxidation Processes for Wastewater Treatment: A Review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Litter, M.I.; Slodowicz, M. An Overview on Heterogeneous Fenton and PhotoFenton Reactions Using Zerovalent Iron Materials. J. Adv. Oxid. Technol. 2017, 20, 20160164. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous Photo-Fenton Processes at near Neutral PH: A Review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Rodríguez-Narváez, O.M.; Pérez, L.S.; Yee, N.G.; Peralta-Hernández, J.M.; Bandala, E.R. Comparison between Fenton and Fenton-like Reactions for l-Proline Degradation. Int. J. Environ. Sci. Technol. 2019, 16, 1515–1526. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A Review on Fenton-like Processes for Organic Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, Z.; Qu, H.; Li, J.; Wang, X.; Li, P.; Liu, H. A New Insight into Fenton and Fenton-like Processes for Water Treatment: Part II. Influence of Organic Compounds on Fe(III)/Fe(II) Interconversion and the Course of Reactions. J. Hazard. Mater. 2013, 250–251, 76–81. [Google Scholar] [CrossRef]
- Lai, L.; He, Y.; Zhou, H.; Huang, B.; Yao, G.; Lai, B. Critical Review of Natural Iron-Based Minerals Used as Heterogeneous Catalysts in Peroxide Activation Processes: Characteristics, Applications and Mechanisms. J. Hazard. Mater. 2021, 416, 125809. [Google Scholar] [CrossRef]
- Kahoush, M.; Behary, N.; Cayla, A.; Nierstrasz, V. Bio-Fenton and Bio-Electro-Fenton as Sustainable Methods for Degrading Organic Pollutants in Wastewater. Process Biochem. 2018, 64, 237–247. [Google Scholar] [CrossRef]
- Ahuja, D.K.; Bachas, L.G.; Bhattacharyya, D. Modified Fenton Reaction for Trichlorophenol Dechlorination by Enzymatically Generated H2O2 and Gluconic Acid Chelate. Chemosphere 2007, 66, 2193–2200. [Google Scholar] [CrossRef]
- Tummino, M.L.; Nisticò, R.; Franzoso, F.; Bianco Prevot, A.; Calza, P.; Laurenti, E.; Paganini, M.C.; Scalarone, D.; Magnacca, G. The “Lab4treat” Outreach Experience: Preparation of Sustainable Magnetic Nanomaterials for Remediation of Model Wastewater. Molecules 2021, 26, 3361. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Ralston, J.; Cao, J.; Jin, X.; Sun, W.; Gao, Z. Efficient Heterogeneous Photodegradation of Eosin Y by Oxidized Pyrite Using the Photo-Fenton Process. Miner. Eng. 2023, 191, 107972. [Google Scholar] [CrossRef]
- Guo, S.; Huang, R.; Yuan, J.; Chen, R.; Chen, F. Efficient Removal of Aromatic Pollutants via Catalytic Wet Peroxide Oxidation over Synthetic Anisotropic Ilmenite/Carbon Nanocomposites. npj Clean Water 2023, 6, 74. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton Catalysts: A Review of Recent Advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Wang, J.; Farias, J.; Tiwary, A.; Tangyie, G.C.; Huddersman, K. Advance Oxidation Process (AOP) of Bisphenol A Using a Novel Surface-Functionalised Polyacrylonitrile (PAN) Fibre Catalyst. Water 2022, 14, 640. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Yang, Z.; Wang, J. Fenton Degradation of 4-Chlorophenol Using H2O2: In Situ Generated by Zn-CNTs/O2 System. RSC Adv. 2017, 7, 49985–49994. [Google Scholar] [CrossRef]
- Zhao, P.; Jin, B.; Zhang, Q.; Peng, R. Graphitic-C3N4 Quantum Dots Modified FeOOH for Photo-Fenton Degradation of Organic Pollutants. Appl. Surf. Sci. 2022, 586, 152792. [Google Scholar] [CrossRef]
- Shi, W.; Sun, W.; Liu, Y.; Zhang, K.; Sun, H.; Lin, X.; Hong, Y.; Guo, F. A Self-Sufficient Photo-Fenton System with Coupling in-Situ Production H2O2 of Ultrathin Porous g-C3N4 Nanosheets and Amorphous FeOOH Quantum Dots. J. Hazard. Mater. 2022, 436, 129141. [Google Scholar] [CrossRef]
- Wang, Y.; Song, H.; Chen, J.; Chai, S.; Shi, L.; Chen, C.; Wang, Y.; He, C. A Novel Solar Photo-Fenton System with Self-Synthesizing H2O2: Enhanced Photo-Induced Catalytic Performances and Mechanism Insights. Appl. Surf. Sci. 2020, 512, 145650. [Google Scholar] [CrossRef]
- Calza, P.; Di Sarro, J.; Magnacca, G.; Bianco Prevot, A.; Laurenti, E. Use of Low-Cost Magnetic Materials Containing Waste Derivatives for the (Photo)-Fenton Removal of Organic Pollutants. Materials 2019, 12, 3942. [Google Scholar] [CrossRef]
- Chavan, R.; Bhat, N.; Parit, S.; Narasimharao, K.; Devan, R.S.; Patil, R.B.; Karade, V.C.; Pawar, N.V.; Kim, J.H.; Jadhav, J.P.; et al. Development of Magnetically Recyclable Nanocatalyst for Enhanced Fenton and Photo-Fenton Degradation of MB and Cr(VI) Photo-Reduction. Mater. Chem. Phys. 2023, 293, 126964. [Google Scholar] [CrossRef]
- Bousalah, D.; Zazoua, H.; Boudjemaa, A.; Benmounah, A.; Messaoud-Boureghda, M.Z.; Bachari, K. Enhanced Reactivity of the CuO-Fe2O3 Intimate Heterojunction for the Oxidation of Quinoline Yellow Dye (E104). Environ. Sci. Pollut. Res. 2022, 29, 69988–69999. [Google Scholar] [CrossRef]
- Mirsadeghi, S.; Zandavar, H.; Rajabi, H.R.; Sajadiasl, F.; Ganjali, M.R.; Pourmortazavi, S.M. Superior Degradation of Organic Pollutants and H2O2 Generation Ability on Environmentally-Sound Constructed Fe3O4-Cu Nanocomposite. J. Mater. Res. Technol. 2021, 14, 808–821. [Google Scholar] [CrossRef]
- Domenzain-Gonzalez, J.; Castro-Arellano, J.J.; Galicia-Luna, L.A.; Rodriguez-Cruz, M.; Hernandez-Lopez, R.T.; Lartundo-Rojas, L. Photocatalytic Membrane Reactor Based on Mexican Natural Zeolite: RB5 Dye Removal by Photo-Fenton Process. J. Environ. Chem. Eng. 2021, 9, 105281. [Google Scholar] [CrossRef]
- Ju, Y.; Li, H.; Wang, Z.; Liu, H.; Huo, S.; Jiang, S.; Duan, S.; Yao, Y.; Lu, X.; Chen, F. Solar-Driven on-Site H2O2 Generation and Tandem Photo-Fenton Reaction on a Triphase Interface for Rapid Organic Pollutant Degradation. Chem. Eng. J. 2022, 430, 133168. [Google Scholar] [CrossRef]
- Chen, Z.; Lian, C.; Huang, K.; Ji, J.; Yan, Q.; Zhang, J.; Xing, M. “Small Amount for Multiple Times” of H2O2 Feeding Way in MoS2-Fex Heterogeneous Fenton for Enhancing Sulfadiazine Degradation. Chin. Chem. Lett. 2022, 33, 1365–1372. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Y.; Zeng, Y.; Luo, K.; Pan, X. Enhanced Decomposition of H2O2 by Molybdenum Disulfide in a Fenton-like Process for Abatement of Organic Micropollutants. Sci. Total Environ. 2020, 732, 139335. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Lee, H.; Lee, D.; Ko, Y.J.; Woo, H.; Lee, J.; Lee, C.; Pham, A.L.T. Activation of Hydrogen Peroxide by a Titanium Oxide-Supported Iron Catalyst: Evidence for Surface Fe(IV) and Its Selectivity. Environ. Sci. Technol. 2020, 54, 15424–15432. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, X.; Han, J.; Gong, H.; Meng, L.; Mei, X.; Sun, Y.; Qi, L.; Gan, L. Degradation of Emerging Contaminants by Sono-Fenton Process with in Situ Generated H2O2 and the Improvement by P25-Mediated Visible Light Irradiation. J. Hazard. Mater. 2020, 391, 122229. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Li, Y.; Li, L.; He, J.; Hong, P.; Zhang, K.; Cai, X.; Kong, L.; Liu, J. Ultrathin Iron-Cobalt Oxide Nanosheets with Enhanced H2O2 Activation Performance for Efficient Degradation of Tetracycline. Appl. Surf. Sci. 2021, 535, 147655. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Liu, L.; Li, J.; Wang, S.; Znad, H.; Liu, S. Magnetic ZnO@Fe3O4 Composite for Self-Generated H2O2 toward Photo-Fenton-like Oxidation of Nitrophenol. Compos. Part B Eng. 2020, 200, 108345. [Google Scholar] [CrossRef]
- Cao, Y.; Ren, Y.; Zhang, J.; Xie, T.; Lin, Y. Activation of H2O2 by Photo-Generated Electrons for Enhanced Visible Light Driven Methylene Blue Degradation with ZnFe2O4/BiVO4 Heterojunction. Opt. Mater. 2021, 121, 111637. [Google Scholar] [CrossRef]
- Deganello, F.; Tummino, M.L.; Calabrese, C.; Testa, M.L.; Avetta, P.; Fabbri, D.; Prevot, A.B.; Montoneri, E.; Magnacca, G. A New, Sustainable LaFeO3 Material Prepared from Biowaste-Sourced Soluble Substances. New J. Chem. 2015, 39, 877–885. [Google Scholar] [CrossRef]
- Bianco Prevot, A.; Fabbri, D.; Bernardini, E.; Deganello, F.; Tummino, M.L.; Magnacca, G. Insights on the Photocatalytic Performances of LaFeO3 Synthesized by Solution Combustion Synthesis. In Materials Science in Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 357–370. ISBN 9780128218594. [Google Scholar]
- Tummino, M.L.; Vineis, C.; Varesano, A.; Liotta, L.F.; Rigoletto, M.; Laurenti, E.; Deganello, F. Sr0.85Ce0.15Fe0.67Co0.33-XCuxO3 Perovskite Oxides: Effect of B-Site Copper Codoping on the Physicochemical, Catalytic and Antibacterial Properties upon UV or Thermal Activation. Front. Environ. Eng. 2023, 2, 1249931. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, M.; Tang, D.; Xu, Y.; Ran, H.; He, J.; Chen, K.; Sun, J. High H2O2 Selectivity and Enhanced Fe2+ Regeneration toward an Effective Electro-Fenton Process Based on a Self-Doped Porous Biochar Cathode. Appl. Catal. B Environ. 2022, 315, 121523. [Google Scholar] [CrossRef]
- Mafo, S.G.M.; Tchuifon, D.R.T.; Ngakou, C.S.; Fotsop, C.G.; Kouteu, P.A.N.; Doungmo, G.; Ndifor-Angwafor, G.N.; Anagho, S.G. Study of the Degradation of Bezaktiv Brilliant Blue by the Fenton Process Using a Prepared Ferromagnetic Activated Carbon from Rubber Seed Hull as Heterogeneous Catalyst. Desalin. Water Treat. 2023, 287, 200–213. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, W.; Yang, D.; Wang, X.; Li, Y.; Gong, C.; Yan, J.; Zhai, J.; Gao, X.; Luo, Y. A Green Solar Photo-Fenton Process for the Degradation of Carbamazepine Using Natural Pyrite and Organic Acid with in-Situ Generated H2O2. Sci. Total Environ. 2021, 784, 147187. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Shen, L.; Yan, R.; Lu, S.; Zhang, Y.; Zhang, X.; Zhang, H. Heterogeneously Activation of H2O2 and Persulfate with Goethite for Bisphenol A Degradation: A Mechanistic Study. Chemosphere 2020, 261, 127715. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Vione, D.; Rivoira, L.; Castiglioni, M.; Beldean-Galea, M.S.; Bruzzoniti, M.C. Feasibility of a Heterogeneous Nanoscale Zero-Valent Iron Fenton-like Process for the Removal of Glyphosate from Water. Molecules 2023, 28, 2214. [Google Scholar] [CrossRef] [PubMed]
- Deganello, F.; Oko, D.N.; Testa, M.L.; La Parola, V.; Tummino, M.L.; Soares, C.O.; Rivera, J.G.; Orozco, G.; Guay, D.; Tavares, A.C. Perovskite-Type Catalysts Prepared by Nanocasting: Effect of Metal Silicates on the Electrocatalytic Activity toward Oxygen Evolution and Reduction Reactions. ACS Appl. Energy Mater. 2018, 1, 2565–2575. [Google Scholar] [CrossRef]
- Huang, J.; Climent, V.; Groß, A.; Feliu, J.M. Understanding Surface Charge Effects in Electrocatalysis. Part 2: Hydrogen Peroxide Reactions at Platinum. Chin. J. Catal. 2022, 43, 2837–2849. [Google Scholar] [CrossRef]
- Farhadian, N.; Liu, S.; Asadi, A.; Shahlaei, M.; Moradi, S. Enhanced Heterogeneous Fenton Oxidation of Organic Pollutant via Fe-Containing Mesoporous Silica Composites: A Review. J. Mol. Liq. 2021, 321, 114896. [Google Scholar] [CrossRef]
- Salunkhe, T.T.; Gurugubelli, T.R.; Babu, B.; Yoo, K. Recent Innovative Progress of Metal Oxide Quantum-Dot-Integrated g-C3N4 (0D-2D) Synergistic Nanocomposites for Photocatalytic Applications. Catalysts 2023, 13, 1414. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Kang, Q.; Wen, L. Efficient Photocatalytic Degradation of Doxycycline by Coupling α-Bi2O3/g-C3N4 Composite and H2O2 under Visible Light. Environ. Res. 2021, 197, 110925. [Google Scholar] [CrossRef]
- Adhikari, S.; Lee, H.H.; Kim, D.-H. Efficient Visible-Light Induced Electron-Transfer in z-Scheme MoO3/Ag/C3N4 for Excellent Photocatalytic Removal of Antibiotics of Both Ofloxacin and Tetracycline. Chem. Eng. J. 2020, 391, 123504. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhang, F.; Ma, B.; Xu, N.; Binnah Junior, L.; Yao, B.; Yang, Q.; Liu, D.; Ma, Z. Remarkably Enhanced Visible-Light Photocatalytic Hydrogen Evolution and Antibiotic Degradation over g-C3N4 Nanosheets Decorated by Using Nickel Phosphide and Gold Nanoparticles as Cocatalysts. Appl. Surf. Sci. 2020, 517, 146187. [Google Scholar] [CrossRef]
- Jourshabani, M.; Asrami, M.R.; Lee, B.-K. An Efficient and Unique Route for the Fabrication of Highly Condensed Oxygen-Doped Carbon Nitride for the Photodegradation of Synchronous Pollutants and H2O2 Production under Ambient Conditions. Appl. Catal. B Environ. 2022, 302, 120839. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Metal-Free Activation of H2O2 by g-C3N4 under Visible Light Irradiation for the Degradation of Organic Pollutants. Phys. Chem. Chem. Phys. 2012, 14, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Liu, Y.-L.; Tong, L.-G. Enhanced Visible Light Photocatalytic Activity of G-C3N4 Assisted by Hydrogen Peroxide. Mater. Res. Express 2018, 5, 046203. [Google Scholar] [CrossRef]
- Li, T.; Ge, L.; Peng, X.; Wang, W.; Zhang, W. Enhanced Degradation of Sulfamethoxazole by a Novel Fenton-like System with Significantly Reduced Consumption of H2O2 Activated by g-C3N4/MgO Composite. Water Res. 2021, 190, 116777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lei, J.; Wang, F.; Wang, L.; Hoffmann, M.R.; Liu, Y.; In, S.-I.; Zhang, J. Carbon Nitride Nanotubes with in Situ Grafted Hydroxyl Groups for Highly Efficient Spontaneous H2O2 Production. Appl. Catal. B Environ. 2021, 288, 119993. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Sampaio, M.J.; Teixo, J.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Photo-Fenton Degradation Assisted by in Situ Generation of Hydrogen Peroxide Using a Carbon Nitride Photocatalyst. J. Water Process Eng. 2020, 37, 101467. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, S.; Feng, X.; Wang, N.; Ola, O.; Zhu, Y. Recent Progress in Multifunctional Graphene-Based Nanocomposites for Photocatalysis and Electrocatalysis Application. Nanomaterials 2023, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Frindy, S.; Sillanpää, M. Synthesis and Application of Novel α-Fe2O3/Graphene for Visible-Light Enhanced Photocatalytic Degradation of RhB. Mater. Des. 2020, 188, 108461. [Google Scholar] [CrossRef]
- Shi, W.; Wang, L.; Wang, J.; Sun, H.; Shi, Y.; Guo, F.; Lu, C. Magnetically Retrievable CdS/Reduced Graphene Oxide/ZnFe2O4 Ternary Nanocomposite for Self-Generated H2O2 towards Photo-Fenton Removal of Tetracycline under Visible Light Irradiation. Sep. Purif. Technol. 2022, 292, 120987. [Google Scholar] [CrossRef]
- Tantubay, K.; Das, P.; Baskey (Sen), M. Hydrogen Peroxide–Assisted Photocatalytic Dye Degradation over Reduced Graphene Oxide Integrated ZnCr2O4 Nanoparticles. Environ. Sci. Pollut. Res. 2022, 29, 17309–17318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Gao, J.; Meng, H.; Chai, S.; Jian, Y.; Shi, L.; Wang, Y.; He, C. Selective Electrochemical H2O2 Generation on the Graphene Aerogel for Efficient Electro-Fenton Degradation of Ciprofloxacin. Sep. Purif. Technol. 2021, 272, 118884. [Google Scholar] [CrossRef]
- Abdelfatah, A.M.; El-Maghrabi, N.; Mahmoud, A.E.D.; Fawzy, M. Synergetic Effect of Green Synthesized Reduced Graphene Oxide and Nano-Zero Valent Iron Composite for the Removal of Doxycycline Antibiotic from Water. Sci. Rep. 2022, 12, 19372. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhou, M.; Song, G.; Du, X.; Lu, X. Efficient H2O2 Generation and Spontaneous ●OH Conversion for In-Situ Phenol Degradation on Nitrogen-Doped Graphene: Pyrolysis Temperature Regulation and Catalyst Regeneration Mechanism. J. Hazard. Mater. 2020, 397, 122681. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhou, M.; Lu, X.; Yang, W.; Ren, G.; Cai, J. Electrochemical Catalytic Mechanism of N-Doped Graphene for Enhanced H2O2 Yield and in-Situ Degradation of Organic Pollutant. Appl. Catal. B Environ. 2019, 245, 583–595. [Google Scholar] [CrossRef]
- Tummino, M.L.; Varesano, A.; Copani, G.; Vineis, C. A Glance at Novel Materials, from the Textile World to Environmental Remediation. J. Polym. Environ. 2023, 31, 2826–2854. [Google Scholar] [CrossRef]
- Umar, E.; Ikram, M.; Haider, J.; Nabgan, W.; Imran, M.; Nazir, G. 3D Graphene-Based Material: Overview, Perspective, Advancement, Energy Storage, Biomedical Engineering and Environmental Applications a Bibliometric Analysis. J. Environ. Chem. Eng. 2023, 11, 110339. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as Potential Sustainable Precursors for Activated Carbon Production: Multiple Applications in Environmental Protection and Energy Storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Catalytic Wet Peroxide Oxidation: A Route towards the Application of Hybrid Magnetic Carbon Nanocomposites for the Degradation of Organic Pollutants. A Review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, P.; Wei, K.; Huang, X.; Zhang, X. Enhanced H2O2 Activation and Sulfamethoxazole Degradation by Fe-Impregnated Biochar. Chem. Eng. J. 2020, 385, 123921. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhang, J.; Qin, Y.; Wu, Y.; Zhou, M.; Sun, J. Efficient Removal of Tetracycline by H2O2 Activated with Iron-Doped Biochar: Performance, Mechanism, and Degradation Pathways. Chin. Chem. Lett. 2022, 33, 2105–2110. [Google Scholar] [CrossRef]
- Bankole, O.M.; Adanlawo, O.S.; Ojubola, K.I.; Adeyemi, F.O.; Achadu, O.J.; Ogunniyi, J.A.; Olaseni, S.E.; Ogunlaja, A.S. Facile Synthesis of Solar Active Charcoal Passivated Ag3PO4 and Their Two-Channel Mechanisms for H2O2 Formation in Aerated Water. J. Mol. Struct. 2024, 1300, 137264. [Google Scholar] [CrossRef]
- Compton, P.; Dehkordi, N.R.; Larese Casanova, P.; Alshawabkeh, A.N. Activated Carbon Modifications for Heterogeneous Fenton-Like Catalysis. J. Chem. Eng. Catal. 2022, 1, 203. [Google Scholar] [PubMed]
- Chen, X.; Wang, L.; Sun, W.; Yang, Z.; Jin, J.; Huang, Y.; Liu, G. Boron Bifunctional Catalysts for Rapid Degradation of Persistent Organic Pollutants in a Metal-Free Electro-Fenton Process: O2 and H2O2 Activation Process. Environ. Sci. Technol. 2023, 57, 15693–15702. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Z.; Feng, Y.; Cui, Q.; Du, C.; Yu, C.; Liang, L.; Zhao, W.; Feng, J.; Sun, J.; et al. Harnessing Selective and Durable Electrosynthesis of H2O2 over Dual-Defective Yolk-Shell Carbon Nanosphere toward on-Site Pollutant Degradation. Appl. Catal. B Environ. 2021, 298, 120572. [Google Scholar] [CrossRef]
- Yang, G.; Mo, S.; Xing, B.; Dong, J.; Song, X.; Liu, X.; Yuan, J. Effective Degradation of Phenol via Catalytic Wet Peroxide Oxidation over N, S, and Fe-Tridoped Activated Carbon. Environ. Pollut. 2020, 258, 113687. [Google Scholar] [CrossRef]
- Fan, X.; Cao, Q.; Meng, F.; Song, B.; Bai, Z.; Zhao, Y.; Chen, D.; Zhou, Y.; Song, M. A Fenton-like System of Biochar Loading Fe–Al Layered Double Hydroxides (FeAl-LDH@BC)/H2O2 for Phenol Removal. Chemosphere 2021, 266, 128992. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Gui, H.; Li, H.; Feng, S.; Li, Q.; Wang, Y. Role of Cu x O-Anchored Pyrolyzed Hydrochars on H2O2 -Activated Degradation of Tetracycline: Effects of Pyrolysis Temperature and PH. Ind. Eng. Chem. Res. 2022, 61, 8847–8857. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, H.; Li, C.; Zhang, H.; Cao, J.; Meng, G. Degradation of Biologically Treated Coking Wastewater over CuOx/PAC, CuOx/GAC, and CuOx/ACF Catalysts under Microwave Irradiation in the Presence of H2O2. J. Environ. Eng. 2020, 146. [Google Scholar] [CrossRef]
- Dong, H.; Zou, Y.; Zhang, K.; Sun, Y.; Hui, B.; Yang, D.; Cai, L.; Li, J. Biomimetic Design of Wood Carbon-Based Heterogeneous Catalysts for Enhanced Organic Pollutants Degradation. Chem. Eng. J. 2023, 451, 138568. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Ding, Y.; Wang, F.; You, H.; Jin, C. Preparation and Properties of Cu-Ni Bimetallic Oxide Catalyst Supported on Activated Carbon for Microwave Assisted Catalytic Wet Hydrogen Peroxide Oxidation for Biologically Pretreated Coal Chemical Industry Wastewater Treatment. Chemosphere 2019, 214, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Dionysiou, D.D.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Stadler, F.J. Highly Efficient Sr/Ce/Activated Carbon Bimetallic Nanocomposite for Photoinduced Degradation of Rhodamine B. Catal. Today 2019, 335, 437–451. [Google Scholar] [CrossRef]
- Valtchev, V.; Mintova, S. Zeolites and MOFs? In Zeolites and Metal-Organic Frameworks; Amsterdam University Press: Amsterdam, The Netherlands, 2018; pp. 13–24. [Google Scholar]
- Rashed, M.N.; Palanisamy, P.N. Introductory Chapter: Adsorption and Ion Exchange Properties of Zeolites for Treatment of Polluted Water. In Zeolites and Their Applications; InTechOpen: London, UK, 2018. [Google Scholar]
- Zhang, W.; Taheri-Ledari, R.; Saeidirad, M.; Qazi, F.S.; Kashtiaray, A.; Ganjali, F.; Tian, Y.; Maleki, A. Regulation of Porosity in MOFs: A Review on Tunable Scaffolds and Related Effects and Advances in Different Applications. J. Environ. Chem. Eng. 2022, 10, 108836. [Google Scholar] [CrossRef]
- Jalali, S.; Ardjmand, M.; Ramavandi, B.; Nosratinia, F. Elimination of Amoxicillin Using Zeolite Y-Sea Salt as a Good Catalyst for Activation of Hydrogen Peroxide: Investigating Degradation Pathway and the Effect of Wastewater Chemistry. J. Environ. Manag. 2022, 302, 114045. [Google Scholar] [CrossRef]
- Edebali, S. Synthesis and Characterization of MIL-101 (Fe) as Efficient Catalyst for Tetracycline Degradation by Using NaBH4: Artificial Neural Network Modeling. Appl. Surf. Sci. Adv. 2023, 18, 100496. [Google Scholar] [CrossRef]
- Liú, D.; Wang, G.; Liŭ, D.; Lin, J.; He, Y.; Li, X.; Li, Z. Photocatalysis Using Zero-Valent Nano-Copper for Degrading Methyl Orange under Visible Light Irradiation. Opt. Mater. 2016, 53, 155–159. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Chen, Y.; Tan, N.; Wang, J. High-Efficient Generation of H2O2 by Aluminum-Graphite Composite through Selective Oxygen Reduction for Degradation of Organic Contaminants. Environ. Sci. Technol. 2020, 54, 14085–14095. [Google Scholar] [CrossRef]
- Tan, N.; Yang, Z.; Gong, X.B.; Wang, Z.R.; Fu, T.; Liu, Y. In Situ Generation of H2O2 Using MWCNT-Al/O2 System and Possible Application for Glyphosate Degradation. Sci. Total Environ. 2019, 650, 2567–2576. [Google Scholar] [CrossRef]
- Lin, C.-C.; Cheng, Y.-J. Effectiveness of Using Nanoscale Zero-Valent Iron and Hydrogen Peroxide in Degrading Sulfamethazine in Water. J. Taiwan Inst. Chem. Eng. 2021, 118, 179–186. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Liu, L.; Lan, Y. Rapid Removal of P-Chloronitrobenzene from Aqueous Solution by a Combination of Ozone with Zero-Valent Zinc. Sep. Purif. Technol. 2015, 151, 318–323. [Google Scholar] [CrossRef]
- Zhong, W.; Peng, Q.; Liu, K.; Tang, X.; Zhang, Y.; Xing, J. Building Cu0/CuFe2O4 Framework to Efficiently Degrade Tetracycline and Improve Utilization of H2O2 in Fenton-like System. Chem. Eng. J. 2023, 474, 145522. [Google Scholar] [CrossRef]
- Devi, L.G.; Srinivas, M.; ArunaKumari, M.L. Heterogeneous Advanced Photo- Fenton Process Using Peroxymonosulfate and Peroxydisulfate in Presence of Zero Valent Metallic Iron: A Comparative Study with Hydrogen Peroxide Photo-Fenton Process. J. Water Process Eng. 2016, 13, 117–126. [Google Scholar] [CrossRef]
- Li, L.; Hu, J.; Shi, X.; Fan, M.; Luo, J.; Wei, X. Nanoscale Zero-Valent Metals: A Review of Synthesis, Characterization, and Applications to Environmental Remediation. Environ. Sci. Pollut. Res. 2016, 23, 17880–17900. [Google Scholar] [CrossRef]
- Wu, Z.; Xiong, Z.; Lai, B. Metal Sulfide-Based Catalysts in Advanced Oxidation Processes for Water Decontamination. Environ. Funct. Mater. 2022, 1, 298–315. [Google Scholar] [CrossRef]
- Bai, X.; Wang, X.; Jia, T.; Guo, L.; Hao, D.; Zhang, Z.; Wu, L.; Zhang, X.; Yang, H.; Gong, Y.; et al. Efficient Degradation of PPCPs by Mo1−xS2−y with S Vacancy at Phase-Junction: Promoted by Innergenerate-H2O2. Appl. Catal. B Environ. 2022, 310, 121302. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. In Photocatalysts—Applications and Attributes; IntechOpen: London, UK, 2019. [Google Scholar]
- Zulfiqar, M.; Sufian, S.; Rabat, N.E.; Mansor, N. Photocatalytic Degradation and Adsorption of Phenol by Solvent-Controlled TiO2 Nanosheets Assisted with H2O2 and FeCl3: Kinetic, Isotherm and Thermodynamic Analysis. J. Mol. Liq. 2020, 308, 112941. [Google Scholar] [CrossRef]
- Popescu, T.; Oktaviani Matei, C.; Culita, D.C.; Maraloiu, V.-A.; Rostas, A.M.; Diamandescu, L.; Iacob, N.; Savopol, T.; Ilas, M.C.; Feder, M.; et al. Facile Synthesis of Low Toxicity Iron Oxide/TiO2 Nanocomposites with Hyperthermic and Photo-Oxidation Properties. Sci. Rep. 2022, 12, 6887. [Google Scholar] [CrossRef]
- dela Rosa, F.M.; Popović, M.; Papac Zjačić, J.; Radić, G.; Kraljić Roković, M.; Kovačić, M.; Farré, M.J.; Genorio, B.; Lavrenčič Štangar, U.; Kušić, H.; et al. Visible-Light Activation of Persulfate or H2O2 by Fe2O3/TiO2 Immobilized on Glass Support for Photocatalytic Removal of Amoxicillin: Mechanism, Transformation Products, and Toxicity Assessment. Nanomaterials 2022, 12, 4328. [Google Scholar] [CrossRef]
- Farhana Jaafar, N.; Farhana Jaafar, N.; Khairuddean, M.; Nordin, N. A Review on Recent Progression of Modifications on Titania Morphology and Its Photocatalytic Performance. Acta Chim. Slov. 2020, 67, 361–374. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Parangi, T.; Mishra, M.K. Titania Nanoparticles as Modified Photocatalysts: A Review on Design and Development. Comments Inorg. Chem. 2019, 39, 90–126. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Noureen, L.; Wang, Q.; Humayun, M.; Shah, W.A.; Xu, Q.; Wang, X. Recent Advances in Structural Engineering of Photocatalysts for Environmental Remediation. Environ. Res. 2023, 219, 115084. [Google Scholar] [CrossRef]
- Covinich, L.; Felissia, F.; Massa, P.; Fenoglio, R.; Area, M.C. Kinetic Modeling of a Heterogeneous Fenton-Type Oxidative Treatment of Complex Industrial Effluent. Int. J. Ind. Chem. 2018, 9, 215–229. [Google Scholar] [CrossRef]
- Bokhari, T.H.; Ahmad, N.; Jilani, M.I.; Saeed, M.; Usman, M.; Haq, A.U.; Rehman, R.; Iqbal, M.; Nazir, A.; Javed, T. UV/H2O2, UV/H2O2/SnO2 and Fe/H2O2 Based Advanced Oxidation Processes for the Degradation of Disperse Violet 63 in Aqueous Medium. Mater. Res. Express 2020, 7, 015531. [Google Scholar] [CrossRef]
- Habib, I.Y.; Burhan, J.; Jaladi, F.; Lim, C.M.; Usman, A.; Kumara, N.T.R.N.; Tsang, S.C.E.; Mahadi, A.H. Effect of Cr Doping in CeO2 Nanostructures on Photocatalysis and H2O2 Assisted Methylene Blue Dye Degradation. Catal. Today 2021, 375, 506–513. [Google Scholar] [CrossRef]
- Valles-Pérez, B.Y.; Badillo-Ávila, M.A.; Torres-Delgado, G.; Castanedo-Pérez, R.; Zelaya-Ángel, O. Photocatalytic Activity of ZnO + CuO Thin Films Deposited by Dip Coating: Coupling Effect between Oxides. J. Sol-Gel Sci. Technol. 2020, 93, 517–526. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Khoshghadam-Pireyousefan, M.; Shokrianfard-Ravasjan, B.; Azadbeh, M.; Rashedi, H.; Dibazar, M.; Mostafaei, A. Synergetic Photocatalytic Effect of High Purity ZnO Pod Shaped Nanostructures with H2O2 on Methylene Blue Dye Degradation. J. Alloys Compd. 2020, 845, 156333. [Google Scholar] [CrossRef]
- Ene, C.D.; Patrinoiu, G.; Munteanu, C.; Ene, R.; Chifiriuc, M.C.; Carp, O. Multifunctional ZnO Materials Prepared by a Versatile Green Carbohydrate-Assisted Combustion Method for Environmental Remediation Applications. Ceram. Int. 2019, 45, 2295–2302. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Ganjali, M.R. CuCO3 and CuO Nanoparticles; Facile Preparation and Evaluation as Photocatalysts. J. Mater. Sci. Mater. Electron. 2018, 29, 9442–9451. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of Modification Strategies towards Enhanced Charge Carrier Separation and Photocatalytic Degradation Activity of Metal Oxide Semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent Advances in Semiconductor Metal Oxides with Enhanced Methods for Solar Photocatalytic Applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- El-Shamy, A.G. Synthesis of New Magnesium Peroxide (MgO2) Nano-Rods for Pollutant Dye Removal and Antibacterial Applications. Mater. Chem. Phys. 2020, 243, 122640. [Google Scholar] [CrossRef]
- Ali, M.; Farooq, U.; Lyu, S.; Sun, Y.; Li, M.; Ahmad, A.; Shan, A.; Abbas, Z. Synthesis of Controlled Release Calcium Peroxide Nanoparticles (CR-NCPs): Characterizations, H2O2 Liberate Performances and Pollutant Degradation Efficiency. Sep. Purif. Technol. 2020, 241, 116729. [Google Scholar] [CrossRef]
- Kowalkińska, M.; Głuchowski, P.; Swebocki, T.; Ossowski, T.; Ostrowski, A.; Bednarski, W.; Karczewski, J.; Zielińska-Jurek, A. Scheelite-Type Wide-Bandgap ABO 4 Compounds (A = Ca, Sr, and Ba; B = Mo and W) as Potential Photocatalysts for Water Treatment. J. Phys. Chem. C 2021, 125, 25497–25513. [Google Scholar] [CrossRef]
- Kong, J.; Yang, T.; Rui, Z.; Ji, H. Perovskite-Based Photocatalysts for Organic Contaminants Removal: Current Status and Future Perspectives. Catal. Today 2019, 327, 47–63. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Bowen, C.; Zhang, P.; Li, Z.; Yuan, Q.; Ren, X.; Deng, L. Spinel Photocatalysts for Environmental Remediation, Hydrogen Generation, CO 2 Reduction and Photoelectrochemical Water Splitting. J. Mater. Chem. A 2018, 6, 11078–11104. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Zhang, L. Constructed 3D Hierarchical Micro-Flowers CoWO4@Bi2WO6 Z-Scheme Heterojunction Catalyzer: Two-Channel Photocatalytic H2O2 Production and Antibiotics Degradation. Chem. Eng. J. 2021, 420, 127639. [Google Scholar] [CrossRef]
- Das, K.C.; Dhar, S.S. Rapid Catalytic Degradation of Malachite Green by MgFe2O4 Nanoparticles in Presence of H2O2. J. Alloys Compd. 2020, 828, 154462. [Google Scholar] [CrossRef]
- Hong, P.; Li, Y.; He, J.; Saeed, A.; Zhang, K.; Wang, C.; Kong, L.; Liu, J. Rapid Degradation of Aqueous Doxycycline by Surface CoFe2O4/H2O2 System: Behaviors, Mechanisms, Pathways and DFT Calculation. Appl. Surf. Sci. 2020, 526, 146557. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Miao, J.; Geng, W.; Long, M. In-Situ Utilization of Piezo-Generated Hydrogen Peroxide for Efficient p-Chlorophenol Degradation by Fe Loading Bismuth Vanadate. Appl. Surf. Sci. 2021, 543, 148791. [Google Scholar] [CrossRef]
- Demircivi, P.; Simsek, E.B. Visible-Light-Enhanced Photoactivity of Perovskite-Type W-Doped BaTiO3 Photocatalyst for Photodegradation of Tetracycline. J. Alloys Compd. 2019, 774, 795–802. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Roeb, M.; Sattler, C. Citric Acid Auto-Combustion Synthesis of Ti-Containing Perovskites via Aqueous Precursors. Solid State Ion. 2018, 315, 92–97. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Yu, J.C. On-Demand Synthesis of H2O2 by Water Oxidation for Sustainable Resource Production and Organic Pollutant Degradation. Angew. Chem. 2020, 132, 20719–20725. [Google Scholar] [CrossRef]
- Li, M.; Han, N.; Zhang, X.; Wang, S.; Jiang, M.; Bokhari, A.; Zhang, W.; Race, M.; Shen, Z.; Chen, R.; et al. Perovskite Oxide for Emerging Photo(Electro)Catalysis in Energy and Environment. Environ. Res. 2022, 205, 112544. [Google Scholar] [CrossRef] [PubMed]
- Nzuzo, Y.; Oseghale, C.O.; Chike-Ekwughe, A.; Maumela, M.; Bingwa, N. Electronic Distribution and Dynamics as Catalytic Descriptors in Heterogeneous Catalysis: A Mini Review. Catal. Commun. 2024, 106901. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbieriková, Z.; Brezová, V. Influence of Crystallinity and OH Surface Density on the Photocatalytic Activity of TiO2 Powders. J. Photochem. Photobiol. A Chem. 2014, 273, 59–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C.; Li, J.; Zhu, Y. Recent Progress in Nonsacrificial H2O2 Generation Using Organic Photocatalysts and In Situ Applications for Environmental Remediation. Acc. Mater. Res. 2024, 5, 76–88. [Google Scholar] [CrossRef]
- García-Ballesteros, S.; García-Negueroles, P.; Amat, A.M.; Arques, A. Humic-Like Substances as Auxiliaries to Enhance Advanced Oxidation Processes. ACS Omega 2022, 7, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
- Bianco Prevot, A.; Testa, M.L.; Laurenti, E.; Tummino, M.L.; Magnacca, G. Soluble Bioorganic Substances from Compost as Photosensitizers for a Sustainable Homogeneous and Heterogeneous Photocatalysis. In Materials Science in Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 589–601. ISBN 9780128218594. [Google Scholar]
- Vallés, I.; Santos Juanes, L.; Amat, A.M.; Palma, D.; Laurenti, E.; Bianco Prevot, A.; Arques, A. Humic Acids as Complexing Agents to Drive Photo-Fenton at Mild PH in Saline Matrices: Process Performance and Mechanistic Studies. J. Environ. Chem. Eng. 2023, 11, 111391. [Google Scholar] [CrossRef]
- Tummino, M.L.; Testa, M.L.; Malandrino, M.; Gamberini, R.; Bianco Prevot, A.; Magnacca, G.; Laurenti, E. Green Waste-Derived Substances Immobilized on SBA-15 Silica: Surface Properties, Adsorbing and Photosensitizing Activities towards Organic and Inorganic Substrates. Nanomaterials 2019, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Testa, M.L.; Tummino, M.L.; Agostini, S.; Avetta, P.; Deganello, F.; Montoneri, E.; Magnacca, G.; Prevot, A.B. Synthesis, Characterization and Environmental Application of Silica Grafted Photoactive Substances Isolated from Urban Biowaste. RSC Adv. 2015, 5, 47920–47927. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Liu, H.Z.; Huang, M.; Wang, J.; Yu, H.Q. Ligand-Assisted Heterogeneous Catalytic H2O2 Activation for Pollutant Degradation: The Trade-off between Coordination Site Passivation and Adjacent Site Activation. Appl. Catal. B Environ. 2023, 330, 122592. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Minguzzi, A.; Rondinini, S.; Vertova, A. Achieving Efficient H2O2 Production by a Visible-Light Absorbing, Highly Stable Photosensitized TiO2. Appl. Catal. B Environ. 2019, 244, 303–312. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, X.; Yi, Z.; Xu, X.; Yang, J.; Zhu, M. Enhanced Reactive Oxidation Species Generation by Ligand-to-Metal-Charge Transfer between Oxygen Vacancy-Rich ZnO Mesocrystal with Ciprofloxacin Pollutants. Appl. Catal. B Environ. 2023, 321, 122033. [Google Scholar] [CrossRef]
- Fei, B.; Yan, Q.; Wang, J.; Liu, Q.; Long, J.; Li, Y.; Shao, K.; Su, Z.; Sun, W. Green Oxidative Degradation of Methyl Orange with Copper(II) Schiff Base Complexes as Photo-Fenton-Like Catalysts. Z. Anorg. Allg. Chem. 2014, 640, 2035–2040. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Fan, M.; Yuan, L.; Zou, X. From Solid-State Metal Alkoxides to Nanostructured Oxides: A Precursor-Directed Synthetic Route to Functional Inorganic Nanomaterials. Inorg. Chem. Front. 2015, 2, 198–212. [Google Scholar] [CrossRef]
- Paris, C.B.; Howe, A.G.; Lewis, R.J.; Hewes, D.; Morgan, D.J.; He, Q.; Edwards, J.K. Impact of the Experimental Parameters on Catalytic Activity When Preparing Polymer Protected Bimetallic Nanoparticle Catalysts on Activated Carbon. ACS Catal. 2022, 12, 4440–4454. [Google Scholar] [CrossRef] [PubMed]
- Köwitsch, I.; Mehring, M. Carbon Nitride Materials: Impact of Synthetic Method on Photocatalysis and Immobilization for Photocatalytic Pollutant Degradation. J. Mater. Sci. 2021, 56, 18608–18624. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Wang, Y.; Li, X.; Wan, M.; Zhang, G.; Leng, F.; Zhang, H. Insights into the Structures, Inhibitors, and Improvement Strategies of Glucose Oxidase. Int. J. Mol. Sci. 2022, 23, 9841. [Google Scholar] [CrossRef] [PubMed]
- Wohlfahrt, G.; Witt, S.; Hendle, J.; Schomburg, D.; Kalisz, H.M.; Hecht, H.-J. 1.8 and 1.9 Å Resolution Structures of the Penicillium Amagasakiense and Aspergillus Niger Glucose Oxidases as a Basis for Modelling Substrate Complexes. Acta Crystallogr. Sect. D Biol. Crystallogr. 1999, 55, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, M.; Du, N.; Li, Z.; Zhang, Y.; Zhang, Q. Fenton-like Reaction of Glucose Oxidase-Glucose@Kaolin Coupled with Green Rust: A Framework Triggering FeIV=O in Refractory Pollutants Degradation. Sep. Purif. Technol. 2022, 301, 122061. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Liu, S.; Li, C.; Yuan, S. Glucose Oxidase Modified Fenton Reactions for In-Situ ROS Generation and Potential Application in Groundwater Remediation. Chemosphere 2020, 253, 126648. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, V.K.; Alanazi, A.K.; Senthil Kumar, P.; Rajendran, D.S.; Chidambaram, A.; Venkataraman, S.; Kumar, V.V.; Rangasamy, G.; Cabana, H.; Abo-Dief, H.M. Cost-Effective, Scalable Production of Glucose Oxidase Using Casuarina Equisetifolia Biomass and Its Application in the Bio-Fenton Oxidation Process for the Removal of Trace Organic Contaminants from Wastewater. Bioresour. Technol. 2023, 377, 128958. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Martínez-Huitle, C.A.; Nidheesh, P.V. An Overview of Chelate Modified Electro-Fenton Processes. J. Environ. Chem. Eng. 2022, 10, 107183. [Google Scholar] [CrossRef]
- Yang, Y.; Ghatge, S.; Ko, Y.; Yoon, Y.; Ahn, J.-H.; Kim, J.J.; Hur, H.-G. Non-Specific Degradation of Chloroacetanilide Herbicides by Glucose Oxidase Supported Bio-Fenton Reaction. Chemosphere 2022, 292, 133417. [Google Scholar] [CrossRef]
- An, S.; Yoon, Y.; Ahn, J.-H.; Kim, D.; Weon, H.-Y.; Kim, Y.; Hur, H.-G.; Yang, Y. Oxidative Degradation of Bisphenol A by Bio-Fenton Reaction Equipped with Glucose Oxidase and Ferric Citrate: Degradation Kinetics and Pathway. J. Environ. Chem. Eng. 2023, 11, 109349. [Google Scholar] [CrossRef]
- Wang, X.; Song, C.; Liu, X.; Zhang, J.; Zhang, Y.; Shi, X.; Kim, D. Bio-Fenton-Assisted Biological Process for Efficient Mineralization of Polycyclic Aromatic Hydrocarbons from the Environment. Processes 2022, 10, 1316. [Google Scholar] [CrossRef]
- Yoon, Y.; Cho, M. Understanding Atrazine Elimination via Treatment of the Enzyme-Based Fenton Reaction: Kinetics, Mechanism, Reaction Pathway, and Metabolites Toxicity. Chemosphere 2024, 349, 140982. [Google Scholar] [CrossRef] [PubMed]
- Ioffe, M.; Kundu, S.; Perez-Lapid, N.; Radian, A. Heterogeneous Fenton Catalyst Based on Clay Decorated with Nano-Sized Amorphous Iron Oxides Prevents Oxidant Scavenging through Surface Complexation. Chem. Eng. J. 2022, 433, 134609. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-Based Fenton-like Catalysts for Water Treatment: Catalytic Mechanisms and Applications. J. Mol. Liq. 2021, 332, 115755. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-Based Fenton-like Catalysts for Water Treatment: Preparation, Characterization and Modification. Chemosphere 2021, 276, 130177. [Google Scholar] [CrossRef] [PubMed]
- Bhave, C.; Shejwalkar, S. A Review on the Synthesis and Applications of Green Rust for Environmental Pollutant Remediation. Int. J. Environ. Sci. Technol. 2018, 15, 1243–1248. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, Y.; Liu, H. Removal of Trichloroethene by Glucose Oxidase Immobilized on Magnetite Nanoparticles. RSC Adv. 2023, 13, 11853–11864. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L.; Zhang, Q.; Li, M.; Zhao, Z.; Lin, B.; Peng, J.; Shen, H.; He, Q. Fenton-like System of UV/Glucose-Oxidase@Kaolin Coupled with Organic Green Rust: UV-Enhanced Enzyme Activity and the Mechanism of UV Synergistic Degradation of Photosensitive Pollutants. Environ. Res. 2024, 247, 118257. [Google Scholar] [CrossRef]
- Karimi, A.; Aghbolaghy, M.; Khataee, A.; Shoa Bargh, S. Use of Enzymatic Bio-Fenton as a New Approach in Decolorization of Malachite Green. Sci. World J. 2012, 2012, 691569. [Google Scholar] [CrossRef]
- Hakala, M. Photoinhibition of Manganese Enzymes: Insights into the Mechanism of Photosystem II Photoinhibition. J. Exp. Bot. 2006, 57, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Ghatge, S.; Yang, Y.; Ko, Y.; Yoon, Y.; Ahn, J.-H.; Kim, J.J.; Hur, H.-G. Degradation of Sulfonated Polyethylene by a Bio-Photo-Fenton Approach Using Glucose Oxidase Immobilized on Titanium Dioxide. J. Hazard. Mater. 2022, 423, 127067. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Lonappan, L.; Touahar, I.; Fonteneau, É.; Vaidyanathan, V.K.; Cabana, H. Evaluation of Bio-Fenton Oxidation Approach for the Remediation of Trichloroethylene from Aqueous Solutions. J. Environ. Manag. 2020, 270, 110899. [Google Scholar] [CrossRef] [PubMed]
- Banci, L. Structural Properties of Peroxidases. J. Biotechnol. 1997, 53, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Sugano, Y.; Sasaki, K.; Shoda, M. CDNA Cloning and Genetic Analysis of a Novel Decolorizing Enzyme, Peroxidase Gene Dyp from Geotrichum Candidum Dec 1. J. Biosci. Bioeng. 1999, 87, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Faraco, V.; Piscitelli, A.; Sannia, G.; Giardina, P. Identification of a New Member of the Dye-Decolorizing Peroxidase Family from Pleurotus Ostreatus. World J. Microbiol. Biotechnol. 2007, 23, 889–893. [Google Scholar] [CrossRef]
- Battistuzzi, G.; Bellei, M.; Bortolotti, C.A.; Sola, M. Redox Properties of Heme Peroxidases. Arch. Biochem. Biophys. 2010, 500, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Nuell, M.J.; Fang, G.H.; Axley, M.J.; Kenigsberg, P.; Hager, L.P. Isolation and Nucleotide Sequence of the Chloroperoxidase Gene from Caldariomyces Fumago. J. Bacteriol. 1988, 170, 1007–1011. [Google Scholar] [CrossRef]
- Conesa, A.; Punt, P.J.; van den Hondel, C.A.M.J.J. Fungal Peroxidases: Molecular Aspects and Applications. J. Biotechnol. 2002, 93, 143–158. [Google Scholar] [CrossRef]
- Al-Maqdi, K.A.; Hisaindee, S.M.; Rauf, M.A.; Ashraf, S.S. Comparative Degradation of a Thiazole Pollutant by an Advanced Oxidation Process and an Enzymatic Approach. Biomolecules 2017, 7, 64. [Google Scholar] [CrossRef]
- Wagenknecht, H.-A.; Woggon, W.-D. Identification of Intermediates in the Catalytic Cycle of Chloroperoxidase. Chem. Biol. 1997, 4, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Piontek, K.; Strittmatter, E.; Ullrich, R.; Gröbe, G.; Pecyna, M.J.; Kluge, M.; Scheibner, K.; Hofrichter, M.; Plattner, D.A. Structural Basis of Substrate Conversion in a New Aromatic Peroxygenase. J. Biol. Chem. 2013, 288, 34767–34776. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Sugano, Y. Unexpected Diversity of Dye-Decolorizing Peroxidases. Biochem. Biophys. Rep. 2023, 33, 101401. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M.; Ullrich, R.; Pecyna, M.J.; Liers, C.; Lundell, T. New and Classic Families of Secreted Fungal Heme Peroxidases. Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [Google Scholar] [CrossRef] [PubMed]
- Catucci, G.; Valetti, F.; Sadeghi, S.J.; Gilardi, G. Biochemical Features of Dye-decolorizing Peroxidases: Current Impact on Lignin Degradation. Biotechnol. Appl. Biochem. 2020, 67, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Sasaki, M.; Tanaka, Y.; Ishimori, K. A Dye-Decolorizing Peroxidase from Vibrio Cholerae. Biochemistry 2015, 54, 6610–6621. [Google Scholar] [CrossRef] [PubMed]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase Enzymes as Green Catalysts for Bioremediation and Biotechnological Applications: A Review. Sci. Total Environ. 2022, 806, 150500. [Google Scholar] [CrossRef] [PubMed]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Laurenti, E. Hybrid Materials for the Removal of Emerging Pollutants in Water: Classification, Synthesis, and Properties. Chem. Eng. J. Adv. 2022, 10, 100252. [Google Scholar] [CrossRef]
- Calza, P.; Zacchigna, D.; Laurenti, E. Degradation of Orange Dyes and Carbamazepine by Soybean Peroxidase Immobilized on Silica Monoliths and Titanium Dioxide. Environ. Sci. Pollut. Res. 2016, 23, 23742–23749. [Google Scholar] [CrossRef]
- Iñarritu, I.; Torres, E.; Topete, A.; Campos-Terán, J. Immobilization Effects on the Photocatalytic Activity of CdS Quantum Dots-Horseradish Peroxidase Hybrid Nanomaterials. J. Colloid Interface Sci. 2017, 506, 36–45. [Google Scholar] [CrossRef]
- Wu, J.; He, T.; Ma, X.; Li, C.; Han, J.; Wang, L.; Dong, H.; Zhang, R.; Wang, Y. A Novel Immobilized Horseradish Peroxidase Platform Driven by Visible Light for the Complete Mineralization of Sulfadiazine in Water. Int. J. Biol. Macromol. 2023, 253, 127239. [Google Scholar] [CrossRef] [PubMed]
- Baynton, K.J.; Bewtra, J.K.; Biswas, N.; Taylor, K.E. Inactivation of Horseradish Peroxidase by Phenol and Hydrogen Peroxide: A Kinetic Investigation. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 1994, 1206, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Nicell, J.A.; Wright, H. A Model of Peroxidase Activity with Inhibition by Hydrogen Peroxide. Enzym. Microb. Technol. 1997, 21, 302–310. [Google Scholar] [CrossRef]
- Hiner, A.N.P.; Hernández-Ruiz, J.; Rodríguez-López, J.N.; Arnao, M.B.; Varón, R.; García-Cánovas, F.; Acosta, M. The Inactivation of Horseradish Peroxidase Isoenzyme AZ by Hydrogen Peroxide: An Example of Partial Resistance Due to the Formation of a Stable Enzyme Intermediate. JBIC J. Biol. Inorg. Chem. 2001, 6, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Chansaenpak, K.; Kamkaew, A.; Lisnund, S.; Prachai, P.; Ratwirunkit, P.; Jingpho, T.; Blay, V.; Pinyou, P. Development of a Sensitive Self-Powered Glucose Biosensor Based on an Enzymatic Biofuel Cell. Biosensors 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Z.; Dai, J.; Zhang, W.; Jiang, Y.; Zhou, A. A Low-Cost Mobile Platform for Whole Blood Glucose Monitoring Using Colorimetric Method. Microchem. J. 2021, 162, 105814. [Google Scholar] [CrossRef]
- Pantić, N.; Prodanović, R.; Đurđić, K.I.; Polović, N.; Spasojević, M.; Prodanović, O. Optimization of Phenol Removal with Horseradish Peroxidase Encapsulated within Tyramine-Alginate Micro-Beads. Environ. Technol. Innov. 2021, 21, 101211. [Google Scholar] [CrossRef]
- Babaei, H.; Ghobadi Nejad, Z.; Yaghmaei, S.; Farhadi, F. Co-Immobilization of Multi-Enzyme Cascade System into the Metal–Organic Frameworks for the Removal of Bisphenol A. Chem. Eng. J. 2023, 461, 142050. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Jiang, X.; Zhang, W.; Wang, Y.; Wang, Y.; Zhang, Y.; Luo, H.; Yao, B.; Huang, H.; et al. Surface Charge Modifications Modulate Glucose Oxidase PH-Activity Profiles for Efficient Gluconic Acid Production. J. Clean. Prod. 2022, 372, 133817. [Google Scholar] [CrossRef]
- Gao, F.; Guo, Y.; Fan, X.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y.; Wang, X. Enhancing the Catalytic Performance of Chloroperoxidase by Co-Immobilization with Glucose Oxidase on Magnetic Graphene Oxide. Biochem. Eng. J. 2019, 143, 101–109. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Li, M.; Wang, X.; Rao, D.; Bai, X.; Shi, K.; Xu, H.; Hou, S.; Yao, H. Co-Immobilized Bienzyme of Horseradish Peroxidase and Glucose Oxidase on Dopamine-Modified Cellulose–Chitosan Composite Beads as a High-Efficiency Biocatalyst for Degradation of Acridine. RSC Adv. 2022, 12, 23006–23016. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Q.; Li, M.; Qin, S.; Zhao, Z.; Lin, B.; Ding, Y.; Xiang, Y.; Li, C. Horseradish Peroxidase (HRP) and Glucose Oxidase (GOX) Based Dual-Enzyme System: Sustainable Release of H2O2 and Its Effect on the Desirable Ping Pong Bibi Degradation Mechanism. Environ. Res. 2023, 229, 115979. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, M.; Karimi, A.; Aghdasinia, H.; Joghataei, M.-T. Oxidase-Peroxidase Sequential Polymerization for Removal of a Dye from Contaminated Water by Horseradish Peroxidase (HRP)/Glucose Oxidase (GOx)/Polyurethane Hybrid Catalyst. Korean J. Chem. Eng. 2017, 34, 2870–2878. [Google Scholar] [CrossRef]
- Pitzalis, F.; Monduzzi, M.; Salis, A. A Bienzymatic Biocatalyst Constituted by Glucose Oxidase and Horseradish Peroxidase Immobilized on Ordered Mesoporous Silica. Microporous Mesoporous Mater. 2017, 241, 145–154. [Google Scholar] [CrossRef]
- Taboada-Puig, R.; Junghanns, C.; Demarche, P.; Moreira, M.T.; Feijoo, G.; Lema, J.M.; Agathos, S.N. Combined Cross-Linked Enzyme Aggregates from Versatile Peroxidase and Glucose Oxidase: Production, Partial Characterization and Application for the Elimination of Endocrine Disruptors. Bioresour. Technol. 2011, 102, 6593–6599. [Google Scholar] [CrossRef] [PubMed]
- Frew, J.E.; Jones, P.; Scholes, G. Spectrophotometric Determination of Hydrogen Peroxide and Organic Hydroperoxides at Low Concentrations in Aqueous Solution. Anal. Chim. Acta 1983, 155, 139–150. [Google Scholar] [CrossRef]
- Sekar, R.; DiChristina, T.J. Microbially Driven Fenton Reaction for Degradation of the Widespread Environmental Contaminant 1,4-Dioxane. Environ. Sci. Technol. 2014, 48, 12858–12867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, J.; Guo, Z.; Tan, H.; Zhu, X. A Simple Colorimetric Method for Determination of Hydrogen Peroxide in Plant Tissues. Plant Growth Regul. 2006, 49, 113–118. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Optimized Enzymatic Colorimetric Assay for Determination of Hydrogen Peroxide (H2O2) Scavenging Activity of Plant Extracts. MethodsX 2015, 2, 283–291. [Google Scholar] [CrossRef]
- Schüttler, S.; Jolmes, L.; Jeß, E.; Tschulik, K.; Golda, J. Validation of in Situ Diagnostics for the Detection of OH and H2O2 in Liquids Treated by a Humid Atmospheric Pressure Plasma Jet. Plasma Process. Polym. 2024, 21, e2300079. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Nogueira, R.F.P.; Gomes Neto, J.A.; Jardim, W.F.; Rohwedder, J.J.R. Sistema de Injeção Em Fluxo Espectrofotométrico Para Monitorar Peróxido de Hidrogênio Em Processo de Fotodegradação Por Reação Foto-Fenton. Quim. Nova 2001, 24, 188–190. [Google Scholar] [CrossRef]
- Nogueira, R.; Oliveira, M.; Paterlini, W. Simple and Fast Spectrophotometric Determination of H2O2 in Photo-Fenton Reactions Using Metavanadate. Talanta 2005, 66, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Thermofisher Scientific AmplexTM Red Hydrogen Peroxide/Peroxidase Assay Kit. Available online: https://www.thermofisher.com/order/catalog/product/A22188?gclid=EAIaIQobChMI1eDRvZrGhAMVx6doCR1c2QCCEAAYASAAEgLFlvD_BwE&ef_id=EAIaIQobChMI1eDRvZrGhAMVx6doCR1c2QCCEAAYASAAEgLFlvD_BwE:G:s&s_kwcid=AL!3652!3!447292198748!!!g!!!10506731179!109642167571&cid=bid (accessed on 13 March 2024).
- Kyoritsu Chemical-Check Lab Packtest Hydrogen Peroxide. Available online: https://en.kyoritsu-lab.co.jp/products/wak_h2o2 (accessed on 13 March 2024).
- Pratsinis, A.; Kelesidis, G.A.; Zuercher, S.; Krumeich, F.; Bolisetty, S.; Mezzenga, R.; Leroux, J.-C.; Sotiriou, G.A. Enzyme-Mimetic Antioxidant Luminescent Nanoparticles for Highly Sensitive Hydrogen Peroxide Biosensing. ACS Nano 2017, 11, 12210–12218. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, L.; You, Q.; Chen, Y. PA-Tb-Cu MOF as Luminescent Nanoenzyme for Catalytic Assay of Hydrogen Peroxide. Biosens. Bioelectron. 2017, 96, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cai, S.; Ren, Q.-Q.; Wen, W.; Zhao, Y.-D. Recent Advances in Electrochemical Sensing for Hydrogen Peroxide: A Review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-P.; Liang, R.-P.; Qiu, J.-D. Facile Synthesis of Fe3O4@Al2O3 Core–Shell Nanoparticles and Their Application to the Highly Specific Capture of Heme Proteins for Direct Electrochemistry. Biosens. Bioelectron. 2011, 26, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- WANG, S.; XIE, F.; LIU, G. Direct Electrochemistry and Electrocatalysis of Heme Proteins on SWCNTs-CTAB Modified Electrodes. Talanta 2009, 77, 1343–1350. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.-S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.; Fernandez-Merino, Á.; del Caño, R.; Sánchez-Obrero, G.; Madueño, R.; Blázquez, M.; Pineda, T. Behind the Optimization of the Sensor Film: Bioconjugation of Triangular Gold Nanoparticles with Hemoproteins for Sensitivity Enhancement of Enzymatic Biosensors. Biosensors 2023, 13, 467. [Google Scholar] [CrossRef]
- Kanwal, A.; Saif, B.; Muhammad, A.; Liu, W.; Liu, J.; Ren, H.; Yang, P.; Lei, Z. Hemoglobin-Promoted Growth of Polyhedral Gold Nanoparticles for the Detection of Glucose, H2O2, and Ascorbic Acid. ACS Appl. Nano Mater. 2023, 6, 4734–4746. [Google Scholar] [CrossRef]
- Elewi, A.S.; Al-Shammaree, S.A.W.; AL Sammarraie, A.K.M.A. Hydrogen Peroxide Biosensor Based on Hemoglobin-Modified Gold Nanoparticles–Screen Printed Carbon Electrode. Sens. Bio-Sens. Res. 2020, 28, 100340. [Google Scholar] [CrossRef]
- Salimi, A.; Sharifi, E.; Noorbakhsh, A.; Soltanian, S. Direct Electrochemistry and Electrocatalytic Activity of Catalase Immobilized onto Electrodeposited Nano-Scale Islands of Nickel Oxide. Biophys. Chem. 2007, 125, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Rui, Q.; Komori, K.; Tian, Y.; Liu, H.; Luo, Y.; Sakai, Y. Electrochemical Biosensor for the Detection of H2O2 from Living Cancer Cells Based on ZnO Nanosheets. Anal. Chim. Acta 2010, 670, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Alzate, M.; Gallego, J.; Orozco, J. Hybrid Nanomaterial/Catalase-Modified Electrode for Hydrogen Peroxide Sensing. J. Electroanal. Chem. 2021, 880, 114826. [Google Scholar] [CrossRef]
- Yagati, A.K.; Ngoc Le, H.T.; Cho, S. Bioelectrocatalysis of Hemoglobin on Electrodeposited Ag Nanoflowers toward H2O2 Detection. Nanomaterials 2020, 10, 1628. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Chen, Q.; Liu, N.; Cheng, H.; Li, T. Metal-Organic Framework (MOF)-Au@Pt Nanoflowers Composite Material for Electrochemical Sensing of H2O2 in Living Cells. J. Electroanal. Chem. 2021, 897, 115603. [Google Scholar] [CrossRef]
- Dang, W.; Sun, Y.; Jiao, H.; Xu, L.; Lin, M. AuNPs-NH2/Cu-MOF Modified Glassy Carbon Electrode as Enzyme-Free Electrochemical Sensor Detecting H2O2. J. Electroanal. Chem. 2020, 856, 113592. [Google Scholar] [CrossRef]
- Ji, J.; Ko, S.Y.; Choi, K.M.; Kwon, Y. Hydrogen Peroxide Sensor Using the Biomimetic Structure of Peroxidase Including a Metal Organic Framework. Appl. Surf. Sci. 2021, 554, 148786. [Google Scholar] [CrossRef]
- Tong, P.; Asif, M.; Ajmal, M.; Aziz, A.; Sun, Y. A Multicomponent Polymer-Metal-Enzyme System as Electrochemical Biosensor for H2O2 Detection. Front. Chem. 2022, 10, 874965. [Google Scholar] [CrossRef]
- Manickam, P.; Vashist, A.; Madhu, S.; Sadasivam, M.; Sakthivel, A.; Kaushik, A.; Nair, M. Gold Nanocubes Embedded Biocompatible Hybrid Hydrogels for Electrochemical Detection of H2O2. Bioelectrochemistry 2020, 131, 107373. [Google Scholar] [CrossRef]
- Matos-Peralta, Y.; Antuch, M. Review—Prussian Blue and Its Analogs as Appealing Materials for Electrochemical Sensing and Biosensing. J. Electrochem. Soc. 2020, 167, 037510. [Google Scholar] [CrossRef]
- Narendra Kumar, A.V.; Harish, S.; Joseph, J.; Phani, K.L. Nix–Fe(1−x)Fe(CN)6 Hybrid Thin Films Electrodeposited on Glassy Carbon: Effect of Tuning of Redox Potentials on the Electrocatalysis of Hydrogen Peroxide. J. Electroanal. Chem. 2011, 659, 128–133. [Google Scholar] [CrossRef]
- Ishizaki, M.; Ohshida, E.; Tanno, H.; Kawamoto, T.; Tanaka, H.; Hara, K.; Kominami, H.; Kurihara, M. H2O2-Sensing Abilities of Mixed-Metal (Fe-Ni) Prussian Blue Analogs in a Wide PH Range. Inorg. Chim. Acta 2020, 502, 119314. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Jiang, J.; Xu, Z.; Liu, M.; Liu, X.; Tong, H.; Liu, Z.; Qian, D. Facile Syntheses of Bimetallic Prussian Blue Analogues (KxM[Fe(CN)6]·nH2O, M=Ni, Co, and Mn) for Electrochemical Determination of Toxic 2-Nitrophenol. Electrochim. Acta 2020, 353, 136579. [Google Scholar] [CrossRef]
- Zhao, H.-C.; Zhang, P.; Li, S.-H.; Luo, H.-X. Cobalt Hexacyanoferrate-Modified Graphene Platform Electrode and Its Electrochemical Sensing toward Hydrogen Peroxide. Chin. J. Anal. Chem. 2017, 45, 830–836. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, Y.; Cheng, T.; Lai, W.-Y.; Huang, W. Uniform Manganese Hexacyanoferrate Hydrate Nanocubes Featuring Superior Performance for Low-Cost Supercapacitors and Nonenzymatic Electrochemical Sensors. Nanoscale 2015, 7, 16012–16019. [Google Scholar] [CrossRef]
- Pakrudheen, I.; Bathinapatla, A.; Murugan, E. Amphiphilic Dendrimer Loaded Prussian Blue Nanoparticle for the Detection of Hydrogen Peroxide. Indian J. Chem. Technol. 2022, 29, 668–677. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Engelbrekt, C.; Hou, C.; Zhu, N.; Chi, Q. Size-Dependent and Self-Catalytic Gold@Prussian Blue Nanoparticles for the Electrochemical Detection of Hydrogen Peroxide. ChemElectroChem 2020, 7, 3818–3823. [Google Scholar] [CrossRef]
- Noël, J.; Médard, J.; Combellas, C.; Kanoufi, F. Prussian Blue Degradation during Hydrogen Peroxide Reduction: A Scanning Electrochemical Microscopy Study on the Role of the Hydroxide Ion and Hydroxyl Radical. ChemElectroChem 2016, 3, 1178–1184. [Google Scholar] [CrossRef]
- Uzuncar, S.; Ozdogan, N.; Ak, M. Enzyme-Free Detection of Hydrogen Peroxide with a Hybrid Transducing System Based on Sodium Carboxymethyl Cellulose, Poly(3,4-Ethylenedioxythiophene) and Prussian Blue Nanoparticles. Anal. Chim. Acta 2021, 1172, 338664. [Google Scholar] [CrossRef]
- Ying, S.; Chen, C.; Wang, J.; Lu, C.; Liu, T.; Kong, Y.; Yi, F. Synthesis and Applications of Prussian Blue and Its Analogues as Electrochemical Sensors. Chempluschem 2021, 86, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Maleki, F.; Khaaki, P.; Kadhom, M.; Kudaibergenov, N.; Khataee, A. Electrochemical-Based Sensing Platforms for Detection of Glucose and H2O2 by Porous Metal–Organic Frameworks: A Review of Status and Prospects. Biosensors 2023, 13, 347. [Google Scholar] [CrossRef]
- Hu, Y.; Hojamberdiev, M.; Geng, D. Recent Advances in Enzyme-Free Electrochemical Hydrogen Peroxide Sensors Based on Carbon Hybrid Nanocomposites. J. Mater. Chem. C 2021, 9, 6970–6990. [Google Scholar] [CrossRef]
- Shamkhalichenar, H.; Choi, J.-W. Review—Non-Enzymatic Hydrogen Peroxide Electrochemical Sensors Based on Reduced Graphene Oxide. J. Electrochem. Soc. 2020, 167, 037531. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensors: A Review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; Chen, B.; Wang, L. A Facile, Nonreactive Hydrogen Peroxide (H2O2) Detection Method Enabled by Ion Chromatography with UV Detector. Anal. Chem. 2017, 89, 11537–11544. [Google Scholar] [CrossRef] [PubMed]
- Tantawi, O.; Baalbaki, A.; El Asmar, R.; Ghauch, A. A Rapid and Economical Method for the Quantification of Hydrogen Peroxide (H2O2) Using a Modified HPLC Apparatus. Sci. Total Environ. 2019, 654, 107–117. [Google Scholar] [CrossRef]
- Chiesa, M.; Giamello, E.; Livraghi, S.; Paganini, M.C.; Polliotto, V.; Salvadori, E. Electron Magnetic Resonance in Heterogeneous Photocatalysis Research. J. Phys. Condens. Matter 2019, 31, 444001. [Google Scholar] [CrossRef]
- Fernández-Castro, P.; Vallejo, M.; San Román, M.F.; Ortiz, I. Insight on the Fundamentals of Advanced Oxidation Processes. Role and Review of the Determination Methods of Reactive Oxygen Species. J. Chem. Technol. Biotechnol. 2015, 90, 796–820. [Google Scholar] [CrossRef]
- Ngo, T.T.; Lenhoff, H.M. A Sensitive and Versatile Chromogenic Assay for Peroxidase and Peroxidase-Coupled Reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef]
- Cao, W.; Ju, P.; Wang, Z.; Zhang, Y.; Zhai, X.; Jiang, F.; Sun, C. Colorimetric Detection of H2O2 Based on the Enhanced Peroxidase Mimetic Activity of Nanoparticles Decorated Ce2(WO4)3 Nanosheets. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 239, 118499. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics and Their Applications in H2O2 and Glucose Detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Masschelein, W.; Denis, M.; Ledent, R. Spectrophotometric determination of residual hydrogen peroxide. Water Sew. Work. 1977, 124, 69–72. [Google Scholar]
- Vaithyanathan, V.K.; Ravi, S.; Leduc, R.; Vaidyanathan, V.K.; Cabana, H. Utilization of Biosolids for Glucose Oxidase Production: A Potential Bio-Fenton Reagent for Advanced Oxidation Process for Removal of Pharmaceutically Active Compounds. J. Environ. Manag. 2020, 271, 110995. [Google Scholar] [CrossRef]
- Kosaka, K.; Yamada, H.; Matsui, S.; Echigo, S.; Shishida, K. Comparison among the Methods for Hydrogen Peroxide Measurements To Evaluate Advanced Oxidation Processes: Application of a Spectrophotometric Method Using Copper(II) Ion and 2,9-Dimethyl-1,10-Phenanthroline. Environ. Sci. Technol. 1998, 32, 3821–3824. [Google Scholar] [CrossRef]
- Bader, H.; Sturzenegger, V.; Hoigné, J. Photometric Method for the Determination of Low Concentrations of Hydrogen Peroxide by the Peroxidase Catalyzed Oxidation of N,N-Diethyl-p-Phenylenediamine (DPD). Water Res. 1988, 22, 1109–1115. [Google Scholar] [CrossRef]
- Shaked, Y.; Armoza-Zvuloni, R. Dynamics of Hydrogen Peroxide in a Coral Reef: Sources and Sinks. J. Geophys. Res. Biogeosci. 2013, 118, 1793–1801. [Google Scholar] [CrossRef]
- Schick, R.; Strasser, I.; Stabel, H.-H. Fluorometric Determination of Low Concentrations of H2O2 in Water: Comparison with Two Other Methods and Application to Environmental Samples and Drinking-Water Treatment. Water Res. 1997, 31, 1371–1378. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced Oxidation Processes: Performance, Advantages, and Scale-up of Emerging Technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Carvalho Neves, L.; Beber de Souza, J.; de Souza Vidal, C.M.; Herbert, L.T.; de Souza, K.V.; Geronazzo Martins, K.; Young, B.J. Phytotoxicity Indexes and Removal of Color, COD, Phenols and ISA from Pulp and Paper Mill Wastewater Post-Treated by UV/H2O2 and Photo-Fenton. Ecotoxicol. Environ. Saf. 2020, 202, 110939. [Google Scholar] [CrossRef]
- Hofman-Caris, R.C.H.M.; Harmsen, D.J.H.; Puijker, L.; Baken, K.A.; Wols, B.A.; Beerendonk, E.F.; Keltjens, L.L.M. Influence of Process Conditions and Water Quality on the Formation of Mutagenic Byproducts in UV/H2O2 Processes. Water Res. 2015, 74, 191–202. [Google Scholar] [CrossRef]
- Rozas, O.; Vidal, C.; Baeza, C.; Jardim, W.F.; Rossner, A.; Mansilla, H.D. Organic Micropollutants (OMPs) in Natural Waters: Oxidation by UV/H2O2 Treatment and Toxicity Assessment. Water Res. 2016, 98, 109–118. [Google Scholar] [CrossRef]
- Lopez-Lopez, C.; Purswani, J.; Martín-Pascual, J.; Martínez-Toledo, M.V.; Muñío, M.M.; Poyatos, J.M. Toxic Effect of H2O2 in H2O2/UV, Photo-Fenton and Heterogeneous Photocatalysis (TiO2/H2O2/UV) Systems to Treat Textile Wastewater. Desalin. Water Treat. 2015, 56, 3044–3053. [Google Scholar] [CrossRef]
- Arshad, R.; Bokhari, T.H.; Javed, T.; Bhatti, I.A.; Rasheed, S.; Iqbal, M.; Nazir, A.; Naz, S.; Khan, M.I.; Khosa, M.K.K.; et al. Degradation Product Distribution of Reactive Red-147 Dye Treated by UV/H2O2/TiO2 Advanced Oxidation Process. J. Mater. Res. Technol. 2020, 9, 3168–3178. [Google Scholar] [CrossRef]
- Tony, M.A.; Lin, L.-S. Iron Coated-Sand from Acid Mine Drainage Waste for Being a Catalytic Oxidant Towards Municipal Wastewater Remediation. Int. J. Environ. Res. 2021, 15, 191–201. [Google Scholar] [CrossRef]
- Ferro, G.; Guarino, F.; Castiglione, S.; Rizzo, L. Antibiotic Resistance Spread Potential in Urban Wastewater Effluents Disinfected by UV/H2O2 Process. Sci. Total Environ. 2016, 560–561, 29–35. [Google Scholar] [CrossRef]
- Rioja, N.; Zorita, S.; Peñas, F.J. Effect of Water Matrix on Photocatalytic Degradation and General Kinetic Modeling. Appl. Catal. B Environ. 2016, 180, 330–335. [Google Scholar] [CrossRef]
- Maniakova, G.; Kowalska, K.; Murgolo, S.; Mascolo, G.; Libralato, G.; Lofrano, G.; Sacco, O.; Guida, M.; Rizzo, L. Comparison between Heterogeneous and Homogeneous Solar Driven Advanced Oxidation Processes for Urban Wastewater Treatment: Pharmaceuticals Removal and Toxicity. Sep. Purif. Technol. 2020, 236, 116249. [Google Scholar] [CrossRef]
- Wu, Y.; Chu, L.; Liu, W.; Jiang, L.; Chen, X.; Wang, Y.; Zhao, Y. The Screening of Metal Ion Inhibitors for Glucose Oxidase Based on the Peroxidase-like Activity of Nano-Fe3O4. RSC Adv. 2017, 7, 47309–47315. [Google Scholar] [CrossRef]
- Vitale, A.A.; Bernatene, E.A.; Pomilio, A.B. Inhibition of Fenton Reaction of Glucose by Alcohols and Tetrahydrofuran in Catalytic Concentrations: Calculation of the Stability Constants of ROH/Fe2+ Complexes. Curr. Phys. Chem. 2022, 12, 76–87. [Google Scholar] [CrossRef]
- Lindsey, M. Inhibited Hydroxyl Radical Degradation of Aromatic Hydrocarbons in the Presence of Dissolved Fulvic Acid. Water Res. 2000, 34, 2385–2389. [Google Scholar] [CrossRef]
- Malvestiti, J.A.; Dantas, R.F. Disinfection of Secondary Effluents by O3, O3/H2O2 and UV/H2O2: Influence of Carbonate, Nitrate, Industrial Contaminants and Regrowth. J. Environ. Chem. Eng. 2018, 6, 560–567. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, J.; Shan, C.; Li, H.; Yin, Y.; Pan, B. Toward Selective Oxidation of Contaminants in Aqueous Systems. Environ. Sci. Technol. 2021, 55, 14494–14514. [Google Scholar] [CrossRef]
- Mahbub, P.; Duke, M. Scalability of Advanced Oxidation Processes (AOPs) in Industrial Applications: A Review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ghafoori, S.; Mehrvar, M.; Chan, P.K. Photoreactor Scale-up for Degradation of Aqueous Poly(Vinyl Alcohol) Using UV/H2O2 Process. Chem. Eng. J. 2014, 245, 133–142. [Google Scholar] [CrossRef]
- Bar-Niv, N.; Azaizeh, H.; Kuc, M.E.; Azerrad, S.; Haj-Zaroubi, M.; Menashe, O.; Kurzbaum, E. Advanced Oxidation Process UV-H2O2 Combined with Biological Treatment for the Removal and Detoxification of Phenol. J. Water Process Eng. 2022, 48, 102923. [Google Scholar] [CrossRef]
- Otálvaro-Marín, H.L.; González-Caicedo, F.; Arce-Sarria, A.; Mueses, M.A.; Crittenden, J.C.; Machuca-Martinez, F. Scaling-up a Heterogeneous H2O2/TiO2/Solar-Radiation System Using the DamkÖhler Number. Chem. Eng. J. 2019, 364, 244–256. [Google Scholar] [CrossRef]
- Pliego, G.; Zazo, J.A.; Garcia-Muñoz, P.; Munoz, M.; Casas, J.A.; Rodriguez, J.J. Trends in the Intensification of the Fenton Process for Wastewater Treatment: An Overview. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2611–2692. [Google Scholar] [CrossRef]
- Bashir, Y.; Raj, R.; Ghangrekar, M.M.; Nema, A.K.; Das, S. Critical Assessment of Advanced Oxidation Processes and Bio-Electrochemical Integrated Systems for Removing Emerging Contaminants from Wastewater. RSC Sustain. 2023, 1, 1912–1931. [Google Scholar] [CrossRef]
- Sgroi, M.; Snyder, S.A.; Roccaro, P. Comparison of AOPs at Pilot Scale: Energy Costs for Micro-Pollutants Oxidation, Disinfection by-Products Formation and Pathogens Inactivation. Chemosphere 2021, 273, 128527. [Google Scholar] [CrossRef]
- Yao, W.; Ur Rehman, S.W.; Wang, H.; Yang, H.; Yu, G.; Wang, Y. Pilot-Scale Evaluation of Micropollutant Abatements by Conventional Ozonation, UV/O3, and an Electro-Peroxone Process. Water Res. 2018, 138, 106–117. [Google Scholar] [CrossRef]
- Mousset, E.; Loh, W.H.; Lim, W.S.; Jarry, L.; Wang, Z.; Lefebvre, O. Cost Comparison of Advanced Oxidation Processes for Wastewater Treatment Using Accumulated Oxygen-Equivalent Criteria. Water Res. 2021, 200, 117234. [Google Scholar] [CrossRef]
- Cañizares, P.; Paz, R.; Sáez, C.; Rodrigo, M.A. Costs of the Electrochemical Oxidation of Wastewaters: A Comparison with Ozonation and Fenton Oxidation Processes. J. Environ. Manag. 2009, 90, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best Available Technologies and Treatment Trains to Address Current Challenges in Urban Wastewater Reuse for Irrigation of Crops in EU Countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.P.; Sarinho, L.; Nunes, M.I. Application of Life Cycle Assessment to Fenton Processes in Wastewater Treatment—A Review. J. Water Process Eng. 2024, 57, 104692. [Google Scholar] [CrossRef]
- Garcia Montano, J.; Ruiz, N.; Munoz, I.; Domenech, X.; Garciahortal, J.; Torrades, F.; Peral, J. Environmental Assessment of Different Photo-Fenton Approaches for Commercial Reactive Dye Removal. J. Hazard. Mater. 2006, 138, 218–225. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Foteinis, S.; Chatzisymeon, E.; Michael-Kordatou, I.; Fatta-Kassinos, D. Life Cycle Assessment of Solar-driven Oxidation as a Polishing Step of Secondary-treated Urban Effluents. J. Chem. Technol. Biotechnol. 2017, 92, 1315–1327. [Google Scholar] [CrossRef]
- Costamagna, M.; Arques, A.; Lo-Iacono-Ferreira, V.G.; Bianco Prevot, A. Environmental Assessment of Solar Photo-Fenton Processes at Mild Condition in the Presence of Waste-Derived Bio-Based Substances. Nanomaterials 2022, 12, 2781. [Google Scholar] [CrossRef]
- Raj, R.; Tripathi, A.; Das, S.; Ghangrekar, M.M. Is Waste-Derived Catalyst Mediated Electro-Fenton a Sustainable Option for Mitigating Emerging Contaminants from Wastewater? Curr. Opin. Environ. Sci. Health 2024, 37, 100523. [Google Scholar] [CrossRef]
- Feijoo, S.; González-Rodríguez, J.; Fernández, L.; Vázquez-Vázquez, C.; Feijoo, G.; Moreira, M.T. Fenton and Photo-Fenton Nanocatalysts Revisited from the Perspective of Life Cycle Assessment. Catalysts 2019, 10, 23. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.J.; Pariente, M.I.; Melero, J.A.; Martínez, F.; Molina, R. Comparative Life Cycle Assessment (LCA) Study of Heterogeneous and Homogenous Fenton Processes for the Treatment of Pharmaceutical Wastewater. J. Clean. Prod. 2016, 124, 21–29. [Google Scholar] [CrossRef]
- Fan, Y.; Ai, Z.; Zhang, L. Design of an Electro-Fenton System with a Novel Sandwich Film Cathode for Wastewater Treatment. J. Hazard. Mater. 2010, 176, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Ting, W.-P.; Lu, M.-C.; Huang, Y.-H. The Reactor Design and Comparison of Fenton, Electro-Fenton and Photoelectro-Fenton Processes for Mineralization of Benzene Sulfonic Acid (BSA). J. Hazard. Mater. 2008, 156, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic Activity of Metals in Heterogeneous Fenton-like Oxidation of Wastewater Contaminants: A Review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Salmerón, I.; Plakas, K.V.; Sirés, I.; Oller, I.; Maldonado, M.I.; Karabelas, A.J.; Malato, S. Optimization of Electrocatalytic H2O2 Production at Pilot Plant Scale for Solar-Assisted Water Treatment. Appl. Catal. B Environ. 2019, 242, 327–336. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a Full-Scale Wastewater Treatment Plant Upgraded with Ozonation and Biological Post-Treatments: Abatement of Micropollutants, Formation of Transformation Products and Oxidation by-Products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Pepe, G.; Campiglia, P.; Palma, V. Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process. Catalysts 2020, 10, 888. [Google Scholar] [CrossRef]









| System | Pollutants | Conditions | Performance | Mechanism and Notes | Ref. |
|---|---|---|---|---|---|
| 1. Fe(III)-functionalized polyacrylonitrile/polypropylene fiber mesh | Bisphenol A (BPA) | Best setup: 75 ppm of BPA, 300 ppm of H2O2, pH = 3, 60 °C. | 100% BPA abatement in <30 min; effects of temperature and pH. Fast degradation in Rotating Catalytic Reactor. | Fenton process; reusable catalyst but poisoning by the intermediates. | [54] |
| 2. Zn-Carbon Nanotubes (CNTs) in the presence of Fe2+ | 4-chlorophenol (4-CP) | Conc. 4-CP = 50 mg L−1; Conc. Fe2+ 20 mg L−1, pH = 2.0. O2 was fed in the reaction mixture (400 mL min−1). | Abatement of 4-CP and TOC: 98.8% and 87.4%, respectively (20 min). When 4-CP was spiked in real wastewater, the abatements of 4-CP and TOC were 47.0% and 45.6%. Effects of pH, Zn-CNTs dosage and Fe2+ amount. | Fenton: in situ generation of a high concentration of H2O2, rapid regeneration of Fe2+ from the reduction of Fe3+ by Zn and high adsorption ability of Zn-CNT towards pollutants. | [55] |
| 3. Graphitic-C3N4 QDs with FeOOH | Tetracycline (TC), p-nitrophenol (PNP), 2,4-dinitrophenol (2,4-DNP) | 500 W Xe lamp with a 420 nm cut-off; addition of H2O2; optimal pH = 7. | Abatement: TC ca. 90% (2 min), PNP 90% (10 min), 2,4-DNP ca. 90% (5 min). Dependence on catalyst/pollutant ratio, pH, conc. H2O2. | Photo-Fenton: main action of •OH with the aid of O2•−and h+; photogenerated e− in CB favored Fe3+/Fe2+ cycling. | [56] |
| 4. Ultrathin porous Graphitic-C3N4 nanosheets with amorphous FeOOH QDs | Oxytetracycline (OTC) | 300 W Xe lamp with a 420 nm cut-off; pH = 7. | Highest efficiency for 20%FeOOH-composite degrading 86.23% of OTC (120 min) and TOC removal of 48.6%. | Photo-Fenton; Graphitic-C3N4 in situ produced H2O2, improving transport of photogenerated e−-h+ pairs; FeOOH generated •OH. | [57] |
| 5. α-Fe2O3/Graphitic-C3N4 | Rhodamine B (RhB), tetracycline hydrochloride (TC-H) | Simulated solar light with 300 W Xe lamp; neutral pH. | Degradation of RhB 96% (90 min) and TC-H 95% (150 min). | Photo-Fenton: effective separation and transfer of photogenerated charge carriers; H2O2 photoproduction on g-C3N4; •OH generation from H2O2 decomposition on α-Fe2O3; O2•− and h+ played supporting role. | [58] |
| 6. Magnetite/maghemite NPs coated with waste-sourced bio-based substances (BBS) | Phenol (PH) | For Fenton: addition of H2O2 5 × 10−4 M; photoactivation by a lamp with max emission at 365 nm; pH = 3.5. | 100% PH degradation (5 min). Reusability allowed by the constant Fe release from NPs. | Fenton and photo-Fenton processes, but higher efficiency in Fenton mode. In photo-Fenton •OH are generated. BBS acted preventing catalyst oxidation and Fe precipitation. | [59] |
| 7. Glucose-mediated Fe3O4 magnetic NPs | Methylene blue (MB), Cr(VI) | For Fenton and photo-Fenton: addition of H2O2; photocatalytic reduction of Cr(VI); photoactivation with vis. light (250 W). | MB degradation at pH 9: 93% in 75 min by photo-Fenton and 92% in 120 min by Fenton process. 100% photoreduction of Cr(VI) in 25 min. Influence of H2O2 concentration, pH, catalyst/pollutant dosage. | Higher efficiency of both Fenton and photo-Fenton ascribed to the novel synthesis method. •OH was detected as primary ROS. The catalyst was stable and reusable. | [60] |
| 8. CuO-Fe2O3 heterojunction | Quinoline Yellow (QY) | Best setup: H2O2 and QY concentrations 27.6 mM and 100 mg L−1, respectively; pH = 3, 40 °C. | 100% QY removal in ca. 60 min. Dependence on pH, conc. H2O2 and dye, catalyst dose, temperature. | Photo-Fenton and Fenton (no strong effect of irradiation); recyclability. | [61] |
| 9. Fe3O4/Cu magnetic NPs prepared using Rosmarinus officinalis leaves aqueous extract | Methyl Orange (MO), imipenem (IMI), imatinib mesylate (IMA) | 300 W Xe lamp with a 420 nm cut-off. | MO, IMI, IMA degradation of 96.6%, 81.8% and 84%, respectively, after 5400 s; TOC decrement. | Photocatalytic process with production of H2O2 and O2●− as main ROS; reusability of the catalyst; beneficial effect of R. officinalis leaves extract. | [62] |
| 10. Mexican Natural Zeolite-based Membrane | Reactive Black 5 (RB5) | Best setup: pH= 3, conc. H2O2 = 3 g L−1, conc. RB5 = 100 mg L−1, added FeCl3 = 0.013 g L−1, LED lamp emission at 405 nm (2.2 W), permeation flux ≅ 467 cm3 m−2 h−1. | 92.3% discoloration in 30 min; progressive TOC decrement. | Photo-Fenton; reusable membranes, but possible Fe leaching. | [63] |
| 11. Triphase MIL-101(Fe)/Graphitic-C3N4/hydrophobic carbon cloth | Methyl Orange (MO), methylene blue (MB), rhodamine B (RhB), rhodamine 6G (Rh6G) | 300 W Xe lamp; dye solution = 10 ppm, pH = 3. Catalyst (size: 2.5 cm2) hydrophilic surface immersed in solution, while the hydrophobic part was exposed to air for O2 supply. | Abatement: MO 99%, MB 99%, RhB 98%, Rh6G 97% (130 min); high reaction rate constant. | Photo-Fenton with photoactivated in situ production of H2O2, promoted by triphase design of the Z-scheme heterojunction with a favored pathway for O2 transfer. Photoinduced e− and h+ separation efficiency; •OH and O2•− were the main ROS. | [64] |
| 12. MoS2-Fex composite | Sulfadiazine (SD) | Best catalyst: MoS2-Fe75; Optimal conditions: H2O2 addition, SD = 10 mg L−1, pH = 6.5. | SD degradation 91.1% (90 min); effect of catalyst dosage, pH, H2O2 feeding way. | Fenton: MoS2-Fex selectivity for 1O2. “Small amount for multiple times” feeding way of H2O2 increased MoS2-Fex stability and SD degradation rate, reducing H2O2 decomposition. Formation of Fe sludge was much reduced than nano-iron powder. Long-term effectiveness of the MoS2-Fex/H2O2 system. | [65] |
| 13. MoS2 in the presence of Fe3+ | Bisphenol A (BPA), benzoic acid (BA), sulfadiazine (SDZ), rhodamine B (RhB), carbamazepine (CBZ), 4-acetamidophenol (APAP), ciprofloxacin (CIP), tetracycline hydrochloride (TC-H) | Optimal system: 1.0 mM H2O2, 0.3 g L−1 MoS2 and 0.15 mM Fe3+, pH = 3.0. | Abatement (60 min): CBZ 65.9%, APAP 79.1%, SDZ 84.1%, BA 86.0%, RhB 90.8%, CIP 92.5%, BPA 93.0%, TC 100%. Effects of pH and concentrations of pollutant, H2O2, Fe3+, MoS2. | Fenton: strong oxidative intermediate Mo6+ peroxo-complexes besides •OH radicals. Stable and reusable catalyst. | [66] |
| 14. TiO2-supported Fe (FeTi-ox) | Acetaminophen (AAP), benzoic acid (BA), carbamazepine (CBZ), phenol (PH) | Conc. H2O2 = 10 mM and conc. pollutant = 5−10 μM, pH = 7. | Degradations (2 h): AAP 100%, PH 55%, while BA and CBZ were not removed. Pollutants selectively reacted with •OH (BA and CBZ) or with both •OH and Fe(IV) (AAP and PH). | Fenton: relevant interaction H2O2-TiO2 forms a peroxo−titania complex Fe(III)-Ti-OOH, which reacted further with H2O to give surface oxidant Fe[IV]-O2+ even in the presence of Cl−, HCO3− ions and organic matter. Reusability of FeTi-ox. | [67] |
| 15. P25 (TiO2) in the presence of US and Fe2+/Fe3+ | Bisphenol A (BPA), sulfadiazine (SDZ) | US at 400 kHz alone or in the presence of P25 under vis. light (LED lamp emitting at 400–630 nm). | With US and Fe2+: >90% degradation of BPA and >80% for SDZ in 30 min. In the presence of Fe2+/Fe3+, US, P25 and vis. light, 100% SDZ abatement in 60 min. | Homogeneous sono-Fenton: in situ generation of H2O2 and •OH. Sono-photo-Fenton: P25 promoted Fe2+/Fe3+ cycling by the photoproduced e−. P25 favored reaction at circumneutral pH and pollutant mineralization. | [68] |
| 16. Iron-cobalt oxide nanosheets (CoFe-ONSs) | Tetracycline (TC) | Conc. TC 50 mg L−1, conc. H2O2 20mM, neutral pH. | TC removal 83.5% (50 min). Effects of catalyst dosage, conc. H2O2, pH, temperature, conc. TC, anions and water sources. | Fenton: •OH were the main ROS. Redox cycles of FeII/FeIII and CoII/CoIII enhanced •OH generation. Negligible Fe ions leaching from catalyst (reusability). | [69] |
| 17. Magnetic ZnO@Fe3O4 composite | p-nitrophenol (p-NP) | Conc. p-NP 35 mg L−1, pH = 3 (optimal); 100 W incandescent lamp (400–1700 nm). | 100% p-NP removal in 60 min. Effect of temperature. | Photo-Fenton: self-generation of H2O2, with primary role of •OH; catalyst reusability. | [70] |
| 18. ZnFe2O4/BiVO4 heterojunction | Methylene blue (MB). | Best composition: 0.15ZnFe2O4/BiVO4.MB conc. 40 mg L−1, eventual addition of H2O2; photoactivation with 300 W Xe lamp. | MB degradation with photocatalytic method (2 h): 83.7%; with Photo-Fenton (1 h): 98.8%. | Photocatalysis: h+ had a main role in degrading MB; photo-Fenton: activation of H2O2 (•OH production) by photogenerated e−; photogenerated carriers separation efficiency. | [71] |
| 19. LaFeO3 prepared from citric acid and LaFeO3 synthesized from waste-sourced bio-based substances | 4-methylphenol (4-MP) and crystal violet (CV) | Conc. pollutant 10 mg L−1; pH = 8–10; 1500 W Xe lamp with a 340 nm cut-off. | LaFeO3 prepared from waste-sourced bio-based substances removed 100% CV and ca. 40% 4-MP. The citric acid-derived LaFeO3 photodegraded 90% of 4-MP and 30% of CV. | Photocatalysis: different LaFeO3 efficiencies were ascribed to different ζ-potentials. A homogeneous photo-Fenton process can occur when LaFeO3 synthesized from waste-derived substances releases Fe and carboxylate ions. | [72,73] |
| 20. Cerium, Cobalt, Copper-doped Strontium Ferrate (SCFCC) | Escherichia Coli | Best material with 20% Cu doping. Bacteria conc. 1.0–3.0 × 105 CFU/mL. SCFCC/inoculum ratio = 1 g/50 mL. Tests in the dark, and after thermo- or UV-activation (max 70 °C heating and UV-A 300 W lamp, respectively). | Max. bacterial removal 55% in the dark, 98% after UV, 40% after thermal activation. | Photo/Thermo-catalysis: formation of H2O2 and •OH. Activity was also influenced by SCFCC metal ions’ redox couples and oxygen vacancies. | [74] |
| 21. Peanut shell-derived biochar (PBC) in the presence of Fe2+ | Bisphenol A (BPA), dimethyl phthalate (DMP), sulfamethoxazole (SMX) | Degradation tests conducted in a three-electrode cell aerated with O2 (0.4 L min−1); voltage applied 0.5–1.1 V; pH = 3.0, conc. Fe2+ 0.2 mM and conc. pollutants 20 mg L−1. | Removal efficiencies of 98–100% within 15 min; mineralization efficiencies of 83–100%. | Electro-Fenton: PBC hierarchical porous structure and defects caused a high surface area, electrical and ionic conductivity. The presence of OOH/C–O–C and N on the surface ensured a high two-electron ORR selectivity for H2O2 production, accelerated Fe2+ regeneration, also enabling •OH accumulation. | [75] |
| 22. Ferromagnetic activated carbon from rubber seed hull | Bezaktiv Brilliant Blue (BBB) | Best setup: pH = 3, conc. H2O2= 17 mol L−1, conc. BBB = 100 mg L−1. | BBB removal > 75% in all conditions (4 h). Effects of pH, conc. H2O2 and pollutant, catalyst dosage. | Fenton; reusable catalyst. | [76] |
| 23. Natural pyrite (FeS2) | Carbamazepine (CBZ) | Addition of tartaric acid (TA), citric acid (CA), ascorbic acid (AA); simulated sunlight (300 W Xe lamp). | No CBZ degradation with pyrite; CBZ abatement: 70%, 60%, 53% in pyrite/TA, pyrite/CA, pyrite/AA systems, respectively under irradiation (30 min). Effect of catalyst dosage, pH, conc. CBZ. | Photo-Fenton; in situ generated H2O2 without extra pH adjustment; organic acids can form complex with Fe in pyrite, promoting Fe(II) dissolution. Upon irradiation, pyrite is excited to generate photo-e−, able to reduce oxygen to produce H2O2 and •OH. | [77] |
| 24. Goethite (α-FeOOH) | Bisphenol A (BPA) | H2O2 addition (1.0 mM), conc. BPA 0.1 mM; reaction allowed under acidic, neutral and weakly alkaline conditions. | BPA degradation 75.9% after 240 min; pH dependency. | Fenton; •OH production. Good structural stability of catalyst; higher efficiency with H2O2 in comparison to persulfate oxidation system due to the limited radical scavenging. | [78] |
| 25. Heterogeneous Nanoscale Zero Valent Ion (n-ZVI) | Glyphosate (GLY) | Eventual addition of H2O2. Optimal pH 3–4. | Up to 87% of GLY removal (30 min) in Fenton mode. Effects of pH, conc. H2O2, n-ZVI dosage. Efficiency also in tap water (100% degradation in 40 min), despite potentially interfering ions. | Adsorption and Fenton (after H2O2 addition). | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigoletto, M.; Laurenti, E.; Tummino, M.L. An Overview of Environmental Catalysis Mediated by Hydrogen Peroxide. Catalysts 2024, 14, 267. https://doi.org/10.3390/catal14040267
Rigoletto M, Laurenti E, Tummino ML. An Overview of Environmental Catalysis Mediated by Hydrogen Peroxide. Catalysts. 2024; 14(4):267. https://doi.org/10.3390/catal14040267
Chicago/Turabian StyleRigoletto, Monica, Enzo Laurenti, and Maria Laura Tummino. 2024. "An Overview of Environmental Catalysis Mediated by Hydrogen Peroxide" Catalysts 14, no. 4: 267. https://doi.org/10.3390/catal14040267
APA StyleRigoletto, M., Laurenti, E., & Tummino, M. L. (2024). An Overview of Environmental Catalysis Mediated by Hydrogen Peroxide. Catalysts, 14(4), 267. https://doi.org/10.3390/catal14040267