Coating SiO2 Support with TiO2 or ZrO2 and Effects on Structure and CO Oxidation Performance of Pt Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Morphology and Pt Dispersion
| Samples | Composition (wt%) | SBET (m2/g) | |||
|---|---|---|---|---|---|
| Pt | Ti | Zr | |||
| ICP/EDS | ICP/EDS | ICP/EDS | |||
| Al2O3 | - | - | - | 160 | |
| SiO2 | - | - | - | 195 | |
| Pt/Al2O3 | 0.60/0.83 | - | - | 142 | |
| Pt/SiO2 | 0.83/1.12 | - | - | 176 | |
| Pt/TiO2-SiO2 | 0.94/0.99 | 5.85/7.02 | - | 193 | |
| Pt/ZrO2-SiO2 | 0.88/0.59 | - | 27.3/28.3 | 118 | |


| Catalyst | Condition b | Atomic pair | CN | R (Å) | σ2 (Å2) |
|---|---|---|---|---|---|
| Pt/Al2O3 | H2 | Pt-Pt | 10.4(4c) | 2.743(5) | 0.011(1) |
| O2 | Pt-Pt | 9.2(4) | 2.758(3) | 0.010(1) | |
| Pt/SiO2 | H2 | Pt-Pt | 10.5(2) | 2.743(1) | 0.012(1) |
| O2 | Pt-Pt | 8.2(2) | 2.758(2) | 0.010(1) | |
| Pt/TiO2-SiO2 | H2 | Pt-Pt | 5.2(2) | 2.651(3) | 0.014(1) |
| O2 | Pt-O | 3.7(1) | 2.001(3) | 0.003(1) | |
| Pt/ZrO2-SiO2 | H2 | Pt-Pt | 5.1(1) | 2.604(2) | 0.012(1) |
| O2 | Pt-O | 3.1(1) | 2.024(2) | 0.004(1) |
2.2. Redox Properties of Supported Pt

2.3. Surface Acidity, Basicity and Sulfur Tolerance

| Catalyst | Relative amount of acidic sites | Relative amount of basic sites | Amount of desorbed sulfur (μmol/gcat) | |
|---|---|---|---|---|
| Pt/Al2O3 | 1 | 1 | 726 | |
| Pt/SiO2 | 0 | 0 | 37 | |
| Pt/TiO2-SiO2 | 0.35 | 0 | 171 | |
| Pt/ZrO2-SiO2 | 0.63 | 0.08 | 368 |

2.4. Hydrothermal Stability of Pt Particles
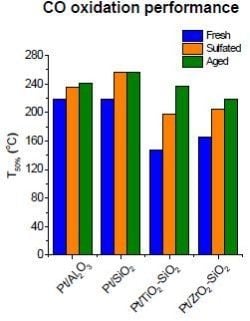
2.5. Catalytic Performance in CO Oxidation

3. Experimental Section
3.1. Preparation of Pt Catalysts
3.2. Characterization
3.3. Evaluation of Catalytic CO Oxidation Performance

4. Conclusions
- Coating SiO2 with TiO2 or ZrO2 via sol-gel method before Pt impregnation led to enhanced dispersion and hydrothermal stability of Pt due to stronger interaction between Pt and supports;
- TiO2 and ZrO2 coatings increased the oxidation state of Pt in O2 environment;
- TiO2 and ZrO2 coatings generated acidity but negligible basicity on the catalyst surface, which explains relatively low and weak sulfur uptake on Pt/TiO2-SiO2 and Pt/ZrO2-SiO2;
- Pt/TiO2-SiO2 and Pt/ZrO2-SiO2 exhibited better CO oxidation performance than Pt/SiO2 and Pt/Al2O3 in fresh, sulfated, and hydrothermally aged states due to the favorable properties brought by metal-oxide coating as described above;
- Results suggest that the sol-gel coating of SiO2 with metal oxides can be an attractive strategy for designing automotive oxidation catalysts with enhanced performance such as low-temperature activity, sulfur tolerance, and hydrothermal stability;
- Further research is necessary to further our understanding of the structure and chemistry of TiO2 and ZrO2 coatings; a follow-up study of Pt/TiO2 and Pt/ZrO2 will be particularly helpful. Furthermore, as Pd is another widely used metal component of state-of-the-art DOCs, it would be appropriate to study Pd catalysts to determine if oxide coating has similarly beneficial impact on catalyst performance.
Acknowledgments
Declaration
Conflict of Interest
References
- Hauff, K.; Tuttlies, U.; Eigenberger, G.; Nieken, U. A global description of DOC kinetics for catalysts with different platinum loadings and aging status. Appl. Catal. B 2010, 100, 10–18. [Google Scholar] [CrossRef]
- Kröcher, O.; Widmer, M.; Elsener, M.; Rothe, D. Adsorption and desorption of SOx on diesel oxidation catalysts. Ind. Eng. Chem. Res. 2009, 48, 9847–9857. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Weng, D. Effects of tungsten oxide on soot oxidation activity and sulfur poisoning resistance of Pt/Al2O3 catalyst. Catal. Sci. Technol. 2011, 1, 644–651. [Google Scholar] [CrossRef]
- Cabello Galisteo, F.; Mariscal, R.; López Granados, M.; Fierro, J.L.G.; Daley, R.A.; Anderson, J.A. Reactivation of sintered Pt/Al2O3 oxidation catalysts. Appl. Catal. B 2005, 59, 227–233. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Kisinger, D.; Abedi, A.; Epling, W.S. Sulfur release from a model Pt/Al2O3 diesel oxidation catalyst: Temperature-programmed and step-response techniques characterization. Appl. Catal. A 2010, 383, 182–191. [Google Scholar]
- Dhakad, M.; Fino, D.; Rayalu, S.S.; Kumar, R.; Watanabe, A.; Haneda, H.; Devotta, S.; Mitsuhashi, T.; Labhsetwar, N. Zirconia supported Ru-Co bimetallic catalysts for diesel soot oxidation. Top. Catal. 2007, 42–43, 273–276. [Google Scholar]
- Kaneeda, M.; Iizuka, H.; Hiratsuka, T.; Shinotsuka, N.; Arai, M. Improvement of thermal stability of NO oxidation Pt/Al2O3 catalyst by addition of Pd. Appl. Catal. B 2009, 90, 564–569. [Google Scholar] [CrossRef]
- Kim, C.H.; Schmid, M.; Schmieg, S.J.; Tan, J.; Li, W. The effect of Pt-Pd ratio on oxidation catalysts under simulated diesel exhaust. SAE Tech. Pap. 2011, 2011-01-1134. [Google Scholar]
- Oi-Uchisawa, J.; Obuchi, A.; Enomoto, R.; Liu, S.; Nanba, T.; Kushiyama, S. Catalytic performance of Pt supported on various metal oxides in the oxidation of carbon black. Appl. Catal. B 2000, 26, 17–24. [Google Scholar]
- Matsumoto, S.; Ikeda, Y.; Suzuki, H.; Ogai, M.; Miyoshi, N. NOx storage-reduction catalyst for automotive exhaust with improved tolerance against sulfur poisoning. Appl. Catal. B 2000, 25, 115–124. [Google Scholar]
- Beutel, T.W.; Dettling, J.C.; Hollobaugh, D.O.; Mueller-Stach, T.W. Pt-Pd diesel oxidation catalyst with CO/HC light-off and HC storage function. U.S. Patent 7 875 573, 25 January 2011. [Google Scholar]
- Kim, M.-Y.; Jung, S.B.; Kim, M.K.; You, Y.S.; Park, J.-H.; Shin, C.-H.; Seo, G. Preparation of highly dispersive and stable platinum catalysts supported on siliceous SBA-15 mesoporous material: Roles of titania layer incorporation and hydrogen peroxide treatment. Catal. Lett. 2009, 129, 194–206. [Google Scholar]
- Kim, M.-Y.; Park, J.-H.; Shin, C.-H.; Han, S.-W.; Seo, G. Dispersion improvement of platinum catalysts supported on silica, silica-alumina and alumina by titania incorporation and pH adjustment. Catal. Lett. 2009, 133, 288–297. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Park, S.M.; Seo, G.; Song, K.-S. Highly stable platinum catalysts in propane combustion prepared by supporting platinum on zirconia-incorporated silica. Catal. Lett. 2010, 138, 205–214. [Google Scholar]
- Kim, M.-Y.; Park, S.M.; Park, J.-H.; Shin, C.-H.; Moon, W.-J.; Sung, N.-E.; Seo, G. Platinum catalysts supported on silicas: Effect of silica characteristics on their catalytic activity in carbon monoxide oxidation. Reac. Kinet. Mech. Catal. 2011, 103, 463–479. [Google Scholar] [CrossRef]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Rad. 2001, 8, 322–324. [Google Scholar] [CrossRef]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 1998, 58, 7565–7576. [Google Scholar]
- Jentys, A. Estimation of mean size and shape of small metal particles by EXAFS. Phys. Chem. Chem. Phys. 1999, 1, 4059–4063. [Google Scholar] [CrossRef]
- De Graaf, J.; van Dillen, A.J.; de Jong, K.P.; Koningsberger, D.C. Preparation of highly dispersed Pt particles in zeolite Y with a narrow particle size distribution: Characterization by hydrogen chemisorption, TEM, EXAFS spectroscopy, and particle modeling. J. Catal. 2001, 203, 307–321. [Google Scholar]
- Lamber, R.; Romanowski, W. Dispersion changes of platinum supported on silica glass during thermal treatment in oxygen and hydrogen atmospheres. J. Catal. 1987, 105, 213–226. [Google Scholar]
- Kamiuchi, N.; Taguchi, K.; Matsui, T.; Kikuchi, R.; Eguchi, K. Sintering and redispersion of platinum catalysts supported on tin oxide. Appl. Catal. B 2009, 89, 65–72. [Google Scholar]
- Wang, T.; Schmidt, L.D. Intraparticle redispersion of Rh and Pt-Rh particles on SiO2 and Al2O3 by oxidation-reduction cycling. J. Catal. 1981, 70, 187–197. [Google Scholar]
- Rickard, J.M.; Genovese, L.; Moata, A.; Nitsche, S. Redispersion of platinum on Pt/Al2O3 model catalyst in oxygen studied by transmission electron microscopy. J. Catal. 1990, 121, 141–152. [Google Scholar]
- Straguzzi, G.I.; Aduriz, H.R.; Gigola, C.E. Redispersion of platinum on alumina support. J. Catal. 1980, 66, 171–183. [Google Scholar]
- Oudenhuijzen, M.K.; Bitter, J.H.; Koningsberger, D.C. The nature of the Pt-H bonding for strongly and weakly bonded hydrogen on platinum. A XAFS spectroscopy study of the Pt-H antibonding shaperesonance and Pt-H EXAFS. J. Phys. Chem. B 2001, 105, 4616–4622. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, L.; Wang, Y.; Zhou, Y.; Gao, Y.; Liu, C.; Xing, W.; Lu, T. Preparation of a carbon supported Pt catalyst using an improved organic sol method and its electrocatalytic activity for methanol oxidation. J. Power Sources 2006, 162, 124–131. [Google Scholar]
- Douidah, A.; Marécot, P.; Szabo, S.; Barbier, J. Evaluation of the metal–support interactions Case of platinum-supported catalysts: Effect of the support nature and the metallic dispersion. Appl. Catal. A 2002, 225, 21–31. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide-support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Kageyama, S.; Seino, S.; Nakagawa, T.; Nitani, H.; Ueno, K.; Daimon, H.; Yamamoto, T.A. Formation of PtRu alloy nanoparticle catalyst by radiolytic process assisted by addition of DL-tartaric acid and its enhanced methanol oxidation activity. J. Nanopart. Res. 2011, 13, 5275–5287. [Google Scholar]
- Yoo, S.J.; Lee, K.-S.; Cho, Y.-H.; Kim, S.-K.; Lim, T.-H.; Sung, Y.-E. Electrocatalytic properties of TiO2-embedded Pt nanoparticles in oxidation of methanol: Particle size effect and proton spillover effect. Electrocatalysis 2011, 2, 297–306. [Google Scholar]
- Yan, W.; Li, Z.; Wei, Z.; Wei, S. Pd-Pt catalysts on fluorinated alumina support studied by X-ray absorption fine structure. In Proceedings of AIP (American Institute of Physics) Conference, Stanford, CA, USA, 9–14 July 2006; 882, pp. 711–713.
- Miller, J.T.; Koningsberger, D.C. The origin of sulfur tolerance in supported platinum catalysts: The relationship between structural and catalytic properties in acidic and alkaline Pt/LTL. J. Catal. 1996, 162, 209–219. [Google Scholar]
- Tanabe, K.; Misono, M.; Ono, Y.; Hattori, H. New solid acids and bases: Their catalytic properties. Stud. Surf. Sci. Catal. 1989, 51, 109–113. [Google Scholar]
- Campbell, C.T.; Ertl, G.; Kuipers, H.; Segner, J. A molecular-beam study of the catalytic-oxidation of CO on a Pt(111) surface. J. Chem. Phys. 1980, 73, 5862–5873. [Google Scholar]
- Bär, M.; Zülicke, C.; Eiswirth, M.; Ertl, G. Theoretical modeling of spatiotemporal self-organization in a surface catalyzed reaction exhibiting bistable kinetics. J. Chem. Phys. 1992, 96, 8595–8604. [Google Scholar] [CrossRef]
- Vannice, M.A.; Hasselbring, L.C.; Sen, B. Direct measurements of heats of adsorption on platinum catalysts. II. CO on Pt dispersed on SiO2, A12O3, SiO2-A12O3, and TiO2. J. Catal. 1986, 97, 66–74. [Google Scholar]
- Bakhmutsky, K.; Wieder, N.L.; Cargnello, M.; Galloway, B.; Fornasiero, P.; Gorte, R.J. versatile route to core-shell catalysts: Synthesis of dispersible M@Oxide (M=Pd, Pt; Oxide=TiO2, ZrO2) nanostructures by self-assembly. ChemSusChem 2012, 5, 140–148. [Google Scholar] [CrossRef]
- Olsson, L.; Karlsson, H. The beneficial effect of SO2 on platinum migration and NO oxidation over Pt containing monolith catalysts. Catal. Today 2009, 147S, S290–S294. [Google Scholar]
- Toops, T.J.; Ottinger, N.A.; Liang, C.; Pihl, J.A.; Payzant, E.A. Impact of dopants on the sulfation, desulfation and NOx reduction performance of Ba-based NOx storage-reduction catalysts. Catal. Today 2011, 160, 131–136. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, M.-Y.; Choi, J.-S.; Toops, T.J.; Jeong, E.-S.; Han, S.-W.; Schwartz, V.; Chen, J. Coating SiO2 Support with TiO2 or ZrO2 and Effects on Structure and CO Oxidation Performance of Pt Catalysts. Catalysts 2013, 3, 88-103. https://doi.org/10.3390/catal3010088
Kim M-Y, Choi J-S, Toops TJ, Jeong E-S, Han S-W, Schwartz V, Chen J. Coating SiO2 Support with TiO2 or ZrO2 and Effects on Structure and CO Oxidation Performance of Pt Catalysts. Catalysts. 2013; 3(1):88-103. https://doi.org/10.3390/catal3010088
Chicago/Turabian StyleKim, Mi-Young, Jae-Soon Choi, Todd J. Toops, Eun-Suk Jeong, Sang-Wook Han, Viviane Schwartz, and Jihua Chen. 2013. "Coating SiO2 Support with TiO2 or ZrO2 and Effects on Structure and CO Oxidation Performance of Pt Catalysts" Catalysts 3, no. 1: 88-103. https://doi.org/10.3390/catal3010088
APA StyleKim, M.-Y., Choi, J.-S., Toops, T. J., Jeong, E.-S., Han, S.-W., Schwartz, V., & Chen, J. (2013). Coating SiO2 Support with TiO2 or ZrO2 and Effects on Structure and CO Oxidation Performance of Pt Catalysts. Catalysts, 3(1), 88-103. https://doi.org/10.3390/catal3010088