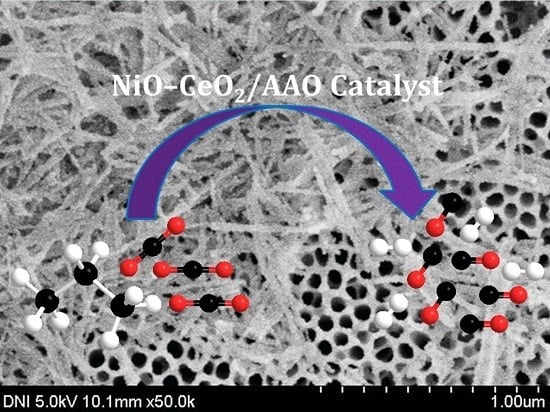
Anodized Aluminum Oxide Supported NiO-CeO2 Catalyst for Dry Reforming of Propane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalyst before Reaction
2.2. Propane/CO2 Reforming
2.3. Characterization of the Catalyst after Reforming
3. Materials and Methods
3.1. Preparation of AAO Support and NiO-CeO2/AAO Catalyst
3.2. Characterizations
3.3. Catalytic Activity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aasberg-Petersen, K.; Dybkjær, I.; Ovesen, C.V.; Schjødt, N.C.; Sehested, J.; Thomsen, S.G. Natural gas to synthesis gas—Catalysts and catalytic processes. J. Nat. Gas Sci. Eng. 2011, 3, 423–459. [Google Scholar] [CrossRef]
- Ye, X.P.; Shoujie, R. Value-added chemicals from glycerol. ACS Symp. Ser. 2014, 1178, 43–80. [Google Scholar]
- Zinoviev, S.; Müller-Langer, F.; Das, P.; Bertero, N.; Fornasiero, P.; Kaltschmitt, M.; Centi, G.; Miertus, S. Next-generation biofuels: Survey of emerging technologies and sustainability issues. ChemSusChem 2010, 3, 1106–1133. [Google Scholar] [CrossRef]
- Althenayan, F.M.; Yei Foo, S.; Kennedy, E.M.; Dlugogorski, B.Z.; Adesina, A.A. Bimetallic Co–Ni/Al2O3 catalyst for propane dry reforming: Estimation of reaction metrics from longevity runs. Chem. Eng. Sci. 2010, 65, 66–73. [Google Scholar] [CrossRef]
- Siahvashi, A.; Chesterfield, D.; Adesina, A.A. Propane CO2 (dry) reforming over bimetallic Mo–Ni/Al2O3 catalyst. Chem. Eng. Sci. 2013, 93, 313–325. [Google Scholar] [CrossRef]
- Siahvashi, A.; Adesina, A.A. Kinetic Study of Propane CO2 Reforming over Bimetallic Mo–Ni/Al2O3 Catalyst. Ind. Eng. Chem. Res. 2013, 52, 15377–15386. [Google Scholar] [CrossRef]
- Yu, Q.; Kong, M.; Liu, T.; Fei, J.; Zheng, X. Non-thermal plasma assisted CO2 reforming of propane over Ni/γ-Al2O3 catalyst. Catal. Commun. 2011, 12, 1318–1322. [Google Scholar] [CrossRef]
- Karuppiah, J.; Mok, Y.S. Plasma-reduced Ni/γ–Al2O3 and CeO2–Ni/γ–Al2O3 catalysts for improving dry reforming of propane. Int. J. Hydrogen Energy 2014, 39, 16329–16338. [Google Scholar] [CrossRef]
- Huang, T.-J.; Wu, C.-Y.; Wang, C.-H. Fuel processing in direct propane solid oxide fuel cell and carbon dioxide reforming of propane over Ni–YSZ. Fuel Proc. Technol. 2011, 92, 1611–1616. [Google Scholar] [CrossRef]
- Adesina, A.A. The role of CO2 in hydrocarbon reforming catalysis: Friend or foe? Curr. Opin. Chem. Eng. 2012, 1, 272–280. [Google Scholar] [CrossRef]
- Gómez-Ramírez, A.; Rico, V.J.; Cotrino, J.; González-Elipe, A.R.; Lambert, R.M. Low temperature production of formaldehyde from carbon dioxide and ethane by plasma-assisted catalysis in a ferroelectrically moderated dielectric barrier discharge reactor. ACS Catal. 2014, 4, 402–408. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Zhao, J.; Wang, L. Thermodynamic analysis of propane dry and steam reforming for synthesis gas or hydrogen production. Int. J. Hydrogen Energy 2010, 35, 12800–12807. [Google Scholar] [CrossRef]
- Siahvashi, A.; Adesina, A.A. Synthesis gas production via propane dry (CO2) reforming: Influence of potassium promotion on bimetallic Mo-Ni/Al2O3. Catal. Today 2013, 214, 30–41. [Google Scholar] [CrossRef]
- Wang, H.Y.; Au, C.T. Carbon dioxide reforming of methane to syngas over SiO2-supported rhodium catalysts. Appl. Catal. A Gen. 1997, 155, 239–252. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Calogero, G.; Torre, T.; Parmaliana, A. Potassium-enhanced stability of Ni/MgO catalysts in the dry-reforming of methane. Catal. Commun. 2001, 2, 49–56. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. Catalytic reforming of methane with carbon dioxide over nickel catalysts I. catalyst characterization and activity. Appl. Catal. A Gen. 1996, 142, 73–96. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, A.; Li, X.; Zhang, S.; Zhu, A.; Ma, Y.; Au, C. Ni-modified Mo2C catalysts for methane dry reforming. Appl. Catal. A Gen. 2012, 431–432, 164–170. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Aika, K.I. Improved resistance against coke deposition of titania supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. Catal. Lett. 2005, 102, 153–157. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Sutthisripok, W.; Assabumrungrat, S. Synthesis gas production from dry reforming of methane over CeO2 doped Ni/Al2O3: Influence of the doping ceria on the resistance toward carbon formation. Chem. Eng. J. 2005, 112, 13–22. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Shen, Y.-M.; Wang, S.-C.; Huang, J.-L. Fabrication of one-dimensional ZnO nanotube and nanowire arrays with an anodic alumina oxide template via electrochemical deposition. Thin Solid Films 2014, 570, 303–309. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, G.-R. Fabrication and characterization of anodic aluminum oxide template. Microelectron. Eng. 2003, 66, 166–170. [Google Scholar] [CrossRef]
- Mamontov, E.; Egami, T.; Brezny, R.; Koranne, M.; Tyagi, S. Lattice defects and oxygen storage capacity of nanocrystalline ceria and ceria-zirconia. J. Phys. Chem. B 2000, 104, 11110–11116. [Google Scholar] [CrossRef]
- Deraz, N.M. Effect of NiO content on structural, surface and catalytic characteristics of nano-crystalline NiO/CeO2 system. Ceram. Int. 2012, 38, 747–753. [Google Scholar] [CrossRef]
- Dobrosz-Gómez, I.; Kocemba, I.; Rynkowski, J.M. Au/Ce1−xZrxO2 as effective catalysts for low-temperature CO oxidation. Appl. Catal. B Environ. 2008, 83, 240–255. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X. Highly active and coking resistant Ni/CeO2–ZrO2 catalyst for partial oxidation of methane. Fuel 2005, 84, 563–567. [Google Scholar] [CrossRef]
- Xu, S.; Yan, X.; Wang, X. Catalytic performances of NiO–CeO2 for the reforming of methane with CO2 and O2. Fuel 2006, 85, 2243–2247. [Google Scholar] [CrossRef]
- Sohier, M.P.; Wrobel, G.; Bonnelle, J.P.; Marcq, J.P. Hydrogenation catalysts based on nickel and rare earths oxides: I. Relation between cations nature, preparation route, hydrogen content and catalytic activity. Appl. Catal. A Gen. 1992, 84, 169–186. [Google Scholar] [CrossRef]
- Lamonier, C.; Ponchel, A.; D’Huysser, A.; Jalowiecki-Duhamel, L. Studies of the cerium-metal–oxygen–hydrogen system (metal = Cu, Ni). Catal. Today 1999, 50, 247–259. [Google Scholar] [CrossRef]
- Solsona, B.; Concepción, P.; Hernández, S.; Demicol, B.; Nieto, J.M.L. Oxidative dehydrogenation of ethane over NiO–CeO2 mixed oxides catalysts. Catal. Today 2012, 180, 51–58. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, A.; Zhang, Y.; Au, C.T.; Yang, X.; Shi, C. Catalytic reduction of NO by CO over NiO/CeO2 catalyst in stoichiometric NO/CO and NO/CO/O2 reaction. Appl. Catal. B Environ. 2008, 81, 141–149. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, X.; Larcher, A.; Xie, K.; Dong, D.; Li, C.-Z. A study on carbon formation over fibrous NiO/CeO2 nanocatalysts during dry reforming of methane. Catal. Today 2013, 216, 44–49. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Boukha, Z.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Behavior of coprecipitated NiAl2O4/Al2O3 catalysts for low-temperature methane steam reforming. Energy Fuels 2014, 28, 7109–7121. [Google Scholar] [CrossRef]
- Si, Z.; Weng, D.; Wu, X.; Ma, Z.; Ma, J.; Ran, R. Lattice oxygen mobility and acidity improvements of NiO–CeO2–ZrO2 catalyst by sulfation for NOx reduction by ammonia. Catal. Today 2013, 201, 122–130. [Google Scholar] [CrossRef]
- Rao, P.V.R.; Kumar, V.P.; Rao, G.S.; Chary, K.V.R. Vapor phase selective hydrogenation of acetone to methyl isobutyl ketone (MIBK) over Ni/CeO2 catalysts. Catal. Sci. Technol. 2012, 2, 1665–1673. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, Z.; Liu, M.; Guo, C.; Sun, P.; Yuan, Z.; Li, B.; Ding, D.; Chen, T. Synthesis and characterization of mesoporous ceria with hierarchical nanoarchitecture controlled by amino acids. J. Phys. Chem. B 2006, 110, 25782–25790. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sun, Y.; Idem, R.O. Comparative study of Ni-based mixed oxide catalyst for carbon dioxide reforming of methane. Energy Fuels 2008, 22, 3575–3582. [Google Scholar] [CrossRef]
- Swaan, H.M.; Kroll, V.C.H.; Martin, G.A.; Mirodatos, C. Deactivation of supported nickel catalysts during the reforming of methane by carbon dioxide. Catal. Today 1994, 21, 571–578. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Bai, P.; Liu, X.; Yan, Z.-F. CO2 reforming of CH4 over nanocrystalline zirconia-supported nickel catalysts. Appl. Catal. B Environ. 2008, 77, 346–354. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Sadanandam, G.; Ramya, K.; Kishore, D.B.; Durgakumari, V.; Subrahmanyam, M.; Chary, K.V.R. A study to initiate development of sustainable Ni/γ-Al2O3 catalyst for hydrogen production from steam reforming of biomass-derived glycerol. RSC Adv. 2014, 4, 32429–32437. [Google Scholar] [CrossRef]
- Carvalho, D.C.; Souza, H.S.A.; Filho, J.M.; Assaf, E.M.; Thyssen, V.V.; Campos, A.; Hernandez, E.P.; Raudel, R.; Oliveira, A.C. Nanosized Pt-containing Al2O3 as an efficient catalyst to avoid coking and sintering in steam reforming of glycerol. RSC Adv. 2014, 4, 61771–61780. [Google Scholar] [CrossRef]
- De Sousa, F.F.; de Sousa, H.S.A.; Oliveira, A.C.; Junior, M.C.C.; Ayala, A.P.; Barros, E.B.; Viana, B.C.; Filho, J.M.; Oliveira, A.C. Nanostructured Ni-containing spinel oxides for the dry reforming of methane: Effect of the presence of cobalt and nickel on the deactivation behaviour of catalysts. Int. J. Hydrogen Energy 2012, 37, 3201–3212. [Google Scholar] [CrossRef]
- Ferencz, Z.; Erdőhelyi, A.; Baán, K.; Oszkó, A.; Óvári, L.; Kónya, Z.; Papp, C.; Steinrück, H.-P.; Kiss, J. Effects of support and Rh additive on Co-based catalysts in the ethanol steam reforming reaction. ACS Catal. 2014, 4, 1205–1218. [Google Scholar] [CrossRef]
- Vári, G.; Óvári, L.; Papp, C.; Steinrück, H.P.; Kiss, J.; Kónya, Z. The interaction of cobalt with CeO2(111) prepared on Cu(111). J. Phys. Chem. C 2015, 119, 9324–9333. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of hydrogen from ethanol: Review of reaction mechanism and catalyst deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef] [PubMed]
- Guil, J.M.; Homs, N.; Llorca, J.; de la Piscina, P.R. Microcalorimetric and infrared studies of ethanol and acetaldehyde adsorption to investigate the ethanol steam reforming on supported cobalt catalysts. J. Phys. Chem. B 2015, 109, 10813–10819. [Google Scholar] [CrossRef] [PubMed]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effects of support modifiers on the catalytic performance of Ni/Al2O3 catalyst in CO2 reforming of methane. Fuel 2014, 129, 197–203. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Herrera, C.; Larrubia, M.Á.; Alemany, L.J. CO2-reforming of natural gas components over a highly stable and selective NiMg/Al2O3 nanocatalyst. Catal. Today 2012, 197, 50–57. [Google Scholar] [CrossRef]
- Robert-Goumet, C.; Monier, G.; Zefack, B.; Chelda, S.; Bideux, L.; Gruzza, B.; Awitor, O.K. SEM and XPS studies of nanohole arrays on InP(1 0 0) surfaces created by coupling AAO templates and low energy Ar+ ion sputtering. Surf. Sci. 2009, 603, 2923–2927. [Google Scholar] [CrossRef]
- Linga Reddy, E.; Karuppiah, J.; Lee, H.C.; Kim, D.H. Steam reforming of methanol over copper loaded anodized aluminum oxide (AAO) prepared through electrodeposition. J. Power Sources 2014, 268, 88–95. [Google Scholar] [CrossRef]
- Li, A.-P.; Müller, F.; Birner, A.; Nielsch, K.; Gösele, U. Fabrication and microstructuring of hexagonally ordered two-dimensional nanopore arrays in anodic alumina. Adv. Mater. 1999, 11, 483–487. [Google Scholar] [CrossRef]
- Linga Reddy, E.; Lee, H.C.; Kim, D.H. Steam reforming of methanol over structured catalysts prepared by electroless deposition of Cu and Zn on anodically oxidized alumina. Int. J. Hydrogen Energy 2015, 40, 2509–2517. [Google Scholar] [CrossRef]
- Mok, Y.S.; Jwa, E.; Hyun, Y.J. Regeneration of C4H10 dry reforming catalyst by nonthermal plasma. J. Energy Chem. 2013, 22, 394–402. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: Hoboken, NJ, USA, 1999; pp. 83–89. [Google Scholar]













| NiO:CeO2 | SBET (m2/g) | NiO | CeO2 | ||
|---|---|---|---|---|---|
| Crystal Lattice Size Strain (nm) Å | Crystal Size (nm) | Lattice Strain Å | |||
| AAO as prepared | 23 | - | - | - | |
| 20%:80% | 15.1 | 9 | 1.1 | 16 | 1.1 |
| 30%:70% | 14.6 | - | - | 23 | 0.8 |
| 40%:60% | 13.9 | 15 | 0.4 | 11 | 1.5 |
| 50%:50% | 12.5 | 14 | 0.1 | 23 | 0.8 |
| 60%:40% | 12.7 | - | - | 18 | 1.0 |
| 70%:30% | 13.1 | 20 | 0.1 | 10 | 1.4 |
| 80%:20% | 12.8 | 31 | 0.4 | - | - |
| Catalyst | Ce 3d5/2 | Ce 3d3/2 | ||||||
|---|---|---|---|---|---|---|---|---|
| υ0 | υ1 | υ2 | υ3 | u0 | u1 | u2 | u3 | |
| 20%NiO-CeO2/AAO | 882.5 | 884.4 | 889.3 | 898.4 | 900.7 | 904.3 | 907.5 | 917.0 |
| 50%NiO-CeO2/AAO | 882.5 | 884.3 | 887.4 | 898.4 | 900.9 | 904.4 | 907.5 | 916.9 |
| 80%NiO-CeO2/AAO | 882.6 | 884.3 | 889.0 | 898.2 | 900.8 | 904.4 | 907.5 | 916.9 |
| Assignments | Ce4+ | Ce3+ | Ce4+ | Ce4+ | Ce4+ | Ce3+ | Ce4+ | Ce4+ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppiah, J.; Linga Reddy, E.; Mok, Y.S. Anodized Aluminum Oxide Supported NiO-CeO2 Catalyst for Dry Reforming of Propane. Catalysts 2016, 6, 154. https://doi.org/10.3390/catal6100154
Karuppiah J, Linga Reddy E, Mok YS. Anodized Aluminum Oxide Supported NiO-CeO2 Catalyst for Dry Reforming of Propane. Catalysts. 2016; 6(10):154. https://doi.org/10.3390/catal6100154
Chicago/Turabian StyleKaruppiah, Jaya, Enakonda Linga Reddy, and Young Sun Mok. 2016. "Anodized Aluminum Oxide Supported NiO-CeO2 Catalyst for Dry Reforming of Propane" Catalysts 6, no. 10: 154. https://doi.org/10.3390/catal6100154
APA StyleKaruppiah, J., Linga Reddy, E., & Mok, Y. S. (2016). Anodized Aluminum Oxide Supported NiO-CeO2 Catalyst for Dry Reforming of Propane. Catalysts, 6(10), 154. https://doi.org/10.3390/catal6100154