Selective Aerobic Oxidation of Benzyl Alcohol Driven by Visible Light on Gold Nanoparticles Supported on Hydrotalcite Modified by Nickel Ion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.1.1. X-ray Diffraction (XRD)
2.1.2. X-ray Photoelectronic Spectra (XPS)
2.1.3. Ultraviolet-Visible Diffuse Reflectance Spectroscopy (UV-Vis DRS)
2.1.4. Atomic Absorption Spectroscopy (AAS)
2.2. Activity Test with Benzyl Alcohol
2.3. Oxidation of Various Alcohols
2.4. Effect of Light Intensity and Light Wavelength
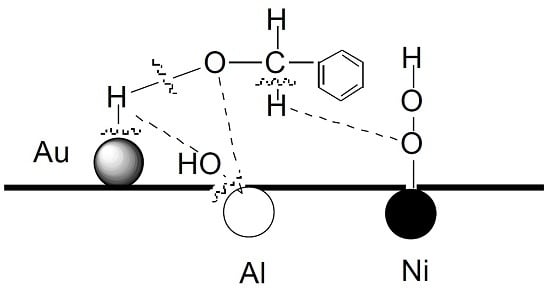
2.5. Reaction Mechanism
2.6. Recyclability Test
3. Experimental Section
3.1. The Preparation of the Catalyst
3.1.1. The Preparation of the Hydrotalcite
3.1.2. The Preparation of Gold Nanoparticle Catalysts
3.2. Catalyst Characterization
3.3. Selective Oxidation of Benzyl Alcohol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dell’Anna, M.M.; Mali, M.; Mastrorilli, P.; Cotugno, P.; Monopoli, A. Oxidation of benzyl alcohols to aldehydes and ketones under air in water using a polymer supported palladium catalyst. J. Mol. Catal. A 2014, 386, 114–119. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Dijksman, A. New developments in catalytic alcohol oxidations for fine chemicals synthesis. Catal. Today 2000, 57, 157–166. [Google Scholar] [CrossRef]
- Tonucci, L.; Nicastro, M.; D’Alessandro, N.; Bressan, M.; D’Ambrosio, P.; Morvillo, A. Catalytic aerobic oxidation of allylic alcohols to carbonyl compounds under mild conditions. Green Chem. 2009, 11, 816–820. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen. Chem. Rev. 2005, 105, 2329–2364. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Res. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Dijksman, A.; Marino-Gonzalez, A.; Payeras, A.M.; Arends, I.W.C.E.; Sheldon, R.A. Efficient and selective aerobic oxidation of alcohols into aldehydes and ketones using ruthenium/TEMPO as the catalytic system. J. Am. Chem. Soc. 2001, 123, 6826–6833. [Google Scholar] [CrossRef] [PubMed]
- Csjernyik, G.; Ell, A.H.; Fadini, L.; Pugin, B.; Backvall, J.E. Efficient ruthenium-catalyzed aerobic oxidation of alcohols using a biomimetic coupled catalytic system. J. Org. Chem. 2002, 67, 1657–1662. [Google Scholar]
- Liu, K.; Yan, X.J.; Zou, P.P.; Wang, Y.Y.; Dai, L.Y. Large size Pd NPs loaded on TiO2 as efficient catalyst for the aerobic oxidation of alcohols to aldehydes. Catal. Commun. 2015, 58, 132–136. [Google Scholar]
- Lien, C.H.; Medlin, J.W. Promotion of activity and selectivity by alkanethiol monolayers for Pd-catalyzed benzyl alcohol hydrodeoxygenation. J. Phys. Chem. C 2014, 118, 23783–23789. [Google Scholar]
- Liu, L.H.; Yu, M.M.; Wayland, B.B.; Fu, X.F. Aerobic oxidation of alcohols catalyzed by rhodium(iii) porphyrin complexes in water: Reactivity and mechanistic studies. Chem. Commun. 2010, 46, 6353–6355. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shoji, T.; Hirano, M. Cationic rhodium(I)/bisphosphane complex-catalyzed isomerization of secondary propargylic alcohols to α,β-enones. Eur. J. Org. Chem. 2007, 2, 2687–2699. [Google Scholar] [CrossRef]
- Gamez, P.; Arends, I.W.; Reedijk, J.; Sheldon, R.A. Copper(II)-catalysed aerobic oxidation of primary alcohols to aldehydes. Chem. Commun. 2003. [Google Scholar] [CrossRef]
- Mitchell, L.J.; Moody, C.J. Solar photochemical oxidation of alcohols using catalytic hydroquinone and copper nanoparticles under oxygen: Oxidative cleavage of lignin models. J. Org. Chem. 2014, 79, 11091–11100. [Google Scholar] [CrossRef]
- Li, Y.; Bian, T.; Du, J.S.; Xiong, Y.L.; Zhan, F.W.; Zhang, H.; Yang, D.R. Facile synthesis of high-quality Pt nanostructures with a controlled aspect ratio for methanol electro-oxidation. Cryst. Eng. Commun. 2014, 16, 8340–8343. [Google Scholar] [CrossRef]
- Frassoldati, A.; Pinel, C.; Besson, M. Promoting effect of water for aliphatic primary and secondary alcohol oxidation over platinum catalysts in dioxane/aqueous solution media. Catal. Today 2011, 173, 81–88. [Google Scholar] [CrossRef]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 2006, 311, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Miedziak, P.; Sankar, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Carley, A.F.; Knight, D.W.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Oxidation of benzyl alcohol using supported gold–palladium nanoparticles. Catal. Today 2011, 164, 315–319. [Google Scholar] [CrossRef]
- Sugano, Y.; Shiraishi, Y.; Tsukamoto, D.; Ichikawa, S.; Tanaka, S.; Hirai, T. Supported Au–Cu bimetallic alloy nanoparticles: An aerobic oxidation catalyst with regenerable activity by visible-light irradiation. Angew. Chem. 2013, 125, 5403–5407. [Google Scholar]
- Huang, X.M.; Wang, X.G.; Wang, X.S.; Wang, X.X.; Tan, M.W.; Ding, W.Z.; Lu, X.G. P123-stabilized Au–Ag alloy nanoparticles for kinetics of aerobic oxidation of benzyl alcohol in aqueous solution. J. Catal. 2013, 301, 217–226. [Google Scholar]
- Kovanda, F.; Jindová, E.; Lang, K.; Kubát, P.; Sedláková, Z. Preparation of layered double hydroxides intercalated with organic anions and their application in LDH/poly(butyl methacrylate) nanocomposites. Appl. Clay Sci. 2010, 48, 260–270. [Google Scholar]
- Choudary, B.M.; Kantam, M.L.; Rahman, A.; Reddy, C.V.; Rao, K.K. The first example of activation of molecular oxygen by nickel in Ni-Al hydrotalcite: A novel protocol for the selective oxidation of alcohols. Angew. Chem. Int. Ed. 2001, 40, 763–766. [Google Scholar]
- Debecker, D.P.; Gaigneaux, E.M.; Busca, G. Exploring, tuning, and exploiting the basicity of hydrotalcites for applications in heterogeneous catalysis. Chem. Eur. J. 2009, 15, 3920–3935. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Chen, X.; Zheng, Z.F.; Ke, X.B.; Jaatinen, E.; Zhao, J.C.; Guo, C.; Xie, T.F.; Wang, D.J. Mechanism of supported gold nanoparticles as photocatalysts under ultraviolet and visible light irradiation. Chem. Commun. 2009. [Google Scholar] [CrossRef]
- Feng, J.; Ma, C.; Miedziak, P.J.; Edwards, J.K.; Brett, G.L.; Li, D.; Du, Y.; Morgan, D.J.; Hutchings, G.J. Au-Pd nanoalloys supported on Mg-Al mixed metal oxides as a multifunctional catalyst for solvent-free oxidation of benzyl alcohol. Dalton Trans. 2013, 42, 14498–14508. [Google Scholar] [CrossRef]
- Fang, W.H.; Chen, J.S.; Zhang, Q.H.; Deng, W.P.; Wang, Y. Hydrotalcite-supported gold catalyst for the oxidant-free dehydrogenation of benzyl alcohol: Studies on support and gold size effects. Chem. Eur. J. 2011, 17, 1247–1256. [Google Scholar] [CrossRef]
- Kawabata, T.; Shinozuka, Y.; Ohishi, Y.; Shishido, T.; Takaki, K.; Takehira, K. Nickel containing Mg-Al hydrotalcite-type anionic clay catalyst for the oxidation of alcohols with molecular oxygen. J. Mol. Catal. A 2005, 236, 206–215. [Google Scholar]
- Yu, J.; Li, J.Y.; Wei, H.L.; Zheng, J.W.; Su, H.Q.; Wang, X.J. Hydrotalcite-supported gold catalysts for a selective aerobic oxidation of benzyl alcohol driven by visible light. J. Mol. Catal. A 2014, 395, 128–136. [Google Scholar]
- Wang, J.; Lang, X.J.; Zhaorigetu, B.; Jia, M.L.; Wang, J.; Guo, X.F.; Zhao, J.C. Aerobic oxidation of alcohols on Au nanocatalyst: Insight to the roles of the Ni–Al layered double hydroxides support. ChemCatChem 2014, 6, 1737–1747. [Google Scholar]
- Kimura, K.; Naya, S.; Jin-Nouchi, Y.; Tada, H. TiO2 crystal form-dependence of the Au/TiO2 plasmon photocatalyst’s activity. J. Phys. Chem. C 2012, 116, 7111–7117. [Google Scholar] [CrossRef]
- Zhang, X.G.; Ke, X.B.; Zhu, H.Y. Zeolite-supported gold nanoparticles for selective photooxidation of aromatic alcohols under visible-light irradiation. Chem. Eur. J. 2012, 18, 8048–8056. [Google Scholar] [CrossRef]
- Wei, H.L.; Li, J.Y.; Yu, J.; Zheng, J.W.; Su, H.Q.; Wang, X.J. Gold nanoparticles supported on metal oxides as catalysts for the direct oxidative esterification of alcohols under mild conditions. Inorg. Chim. Acta 2015, 427, 33–40. [Google Scholar] [CrossRef]
- Heidarnezhad, A.; Zamani, F. Chromium containing Fe3O4/polyacrylonitrile–ethylenediamine as a magnetically recoverable catalyst for alcohol oxidation. Catal. Commun. 2015, 60, 105–109. [Google Scholar] [CrossRef]
- Conte, M.; Miyamura, H.; Kobayashi, S.; Chechik, V. Spin trapping of Au-H intermediate in the alcohol oxidation by supported and unsupported gold catalysts. J. Am. Chem. Soc. 2009, 131, 7189–7196. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, C.; Hensen, E.J.M. Efficient tandem synthesis of methyl esters and imines by using versatile hydrotalcite-supported gold nanoparticles. Chem. Eur. J. 2012, 18, 12122–12129. [Google Scholar] [CrossRef] [PubMed]






| Sample | Chemical Composition (wt. %) | |||
|---|---|---|---|---|
| Au/Ni3Al HT | Ni2+ | Al3+ | Au | Ni2+:Al3+ molar ratio |
| 40.5 | 5.8 | 2.6 | 3.3 | |
| Entry | Catalyst | Conversion (%) b | Selectivity (%) b | ||
|---|---|---|---|---|---|
| Light | Dark | Light | Dark | ||
| 1 | MgAl HT | 14.9 | 0.2 | >99 | >99 |
| 2 | Ni0.3Mg0.7Al HT | 4.7 | 2.6 | >99 | >99 |
| 3 | Ni0.5Mg0.5Al HT | 4.1 | 1.2 | >99 | >99 |
| 4 | Ni0.7Mg0.3Al HT | 7.7 | 0.4 | >99 | >99 |
| 5 | NiAl HT | 1.4 | 0.3 | >99 | >99 |
| 6 | Ni3Al HT | 6.5 | 4.5 | >99 | >99 |
| 7 | Ni5Al HT | 5.6 | 4.5 | >99 | >99 |
| 8 | Au/MgAl HT | 31.2 | 5.0 | >99 | >99 |
| 9 | Au/Ni0.3Mg0.7Al HT | 28.4 | 11.2 | >99 | >99 |
| 10 | Au/Ni0.5Mg0.5Al HT | 31.4 | 1.2 | >99 | >99 |
| 11 | Au/Ni0.7Mg0.3Al HT | 26.2 | 2.3 | >99 | >99 |
| 12 | Au/NiAl HT | 20.9 | 2.3 | >99 | >99 |
| 13 | Au/Ni3Al HT | 41.1 | 6.8 | >99 | >99 |
| 14 | Au/Ni5Al HT | 37.3 | 5.9 | >99 | >99 |
| 15 | 0.5 wt % Au/Ni3Al HT | 19.1 | 0.5 | >99 | >99 |
| 16 | 1 wt % Au/Ni3Al HT | 19.6 | 0.4 | >99 | >99 |
| 17 | 2 wt % Au/Ni3Al HT | 26.7 | 3.5 | >99 | >99 |
| 18 | 4 wt % Au/Ni3Al HT | 17.7 | 7.8 | >99 | >99 |
| Entry | Solvent | Conversion (%) b | Selectivity (%) b | ||
|---|---|---|---|---|---|
| Light | Dark | Light | Dark | ||
| 1 | toluene | 41.8 | 14.7 | >99 | >99 |
| 2 | benzotrifluoride | 74.5 (45.0) c | 21.7 (3.9) c | >99 | >99 |
| 3 | 1, 4-dioxane | 68.6 (41.1) c | 24.8 (6.8) c | >99 | >99 |
| 4 | petroleum ether | 47.7 | 20.3 | 59.2 d | 80.2 d |
| 5 | DMSO | 5.9 | 1.8 | >99 | >99 |
| 6 | DMF | 23.6 | 11.6 | >99 | >99 |
| 7 | ethanol | 59.7 | 13.3 | >99 | >99 |
| 8 | methanol | 22.2 | 9.9 | 53.7 e | 34.7 e |
| Entry | Substrate | Product | Conversion (%) b | Selectivity (%) b | ||
|---|---|---|---|---|---|---|
| Light | Dark | Light | Dark | |||
| 1 | benzyl alcohol | benzaldehyde | 74.5 | 21.7 | >99 | >99 |
| 2 | 4-methyl benzyl alcohol | 4-methyl benzaldehyde | 60.1 | 27.2 | >99 | >99 |
| 3 | 4-methoxybenzylalcohol | 4-methoxy benzaldehyde | 77.8 | 34.4 | >99 | >99 |
| 4 c | 4-nitro benzyl alcohol | 4-nitrobenzaldehyde | 21.4 | 5.1 | >99 | >99 |
| 5 | 4-chloro benzyl alcohol | 4-chlorobenzaldehyde | 62.7 | 18.8 | >99 | >99 |
| 6 | phenylethanol | acetophenone | 89.1 | 9.1 | >99 | >99 |
| 7 | cinnamyl alcohol | cinnamyl aldehyde | 67.7 | 5.8 | >99 | >99 |
| 8 | crotyl alcohol | crotonaldehyde | 32.1 | 17.0 | >99 | >99 |
| 9 | n-octanol | n-octanal | 4.8 | 0.3 | >99 | >99 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Wang, Y.; Zhao, P.; Bai, M.; Xin, H.; Guo, Z.; Li, J. Selective Aerobic Oxidation of Benzyl Alcohol Driven by Visible Light on Gold Nanoparticles Supported on Hydrotalcite Modified by Nickel Ion. Catalysts 2016, 6, 64. https://doi.org/10.3390/catal6050064
Guo D, Wang Y, Zhao P, Bai M, Xin H, Guo Z, Li J. Selective Aerobic Oxidation of Benzyl Alcohol Driven by Visible Light on Gold Nanoparticles Supported on Hydrotalcite Modified by Nickel Ion. Catalysts. 2016; 6(5):64. https://doi.org/10.3390/catal6050064
Chicago/Turabian StyleGuo, Dapeng, Yan Wang, Peng Zhao, Meifen Bai, Hui Xin, Zhi Guo, and Jingyi Li. 2016. "Selective Aerobic Oxidation of Benzyl Alcohol Driven by Visible Light on Gold Nanoparticles Supported on Hydrotalcite Modified by Nickel Ion" Catalysts 6, no. 5: 64. https://doi.org/10.3390/catal6050064
APA StyleGuo, D., Wang, Y., Zhao, P., Bai, M., Xin, H., Guo, Z., & Li, J. (2016). Selective Aerobic Oxidation of Benzyl Alcohol Driven by Visible Light on Gold Nanoparticles Supported on Hydrotalcite Modified by Nickel Ion. Catalysts, 6(5), 64. https://doi.org/10.3390/catal6050064