Enhancement of Degradation and Dechlorination of Trichloroethylene via Supporting Palladium/Iron Bimetallic Nanoparticles onto Mesoporous Silica
Abstract
:1. Introduction
2. Results
2.1. Physical Characterization
2.2. Enhancement of Degradation and Dechlorination of Trichloroethylene
3. Discussion
3.1. Pore Structure and Dispersion of Pd/Fe Nanoparticles
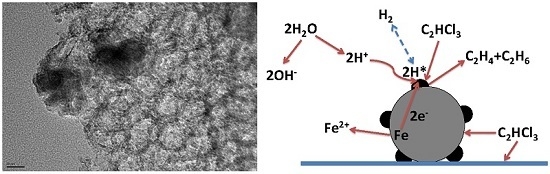
3.2. Enhancement of Degradation and Dechlorination of Trichloroethylene
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Mesoporous Silica
4.2.2. Preparation of Pd/Fe Nanoparticles Supported on Mesoporous Silica and Bare Pd/Fe Nanoparticles
4.2.3. Characterization of Mesoporous Silica, Pd/Fe Nanoparticles Supported on Mesoporous Silica and Bare Pd/Fe Nanoparticles
4.2.4. Degradation and Dechlorination of Trichloroethylene
4.2.5. Sample Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix



References
- Chun, L.C.; Baer, D.R.; Matson, D.W.; Amonette, J.E.; Penn, R.L. Characterization and reactivity of iron nanoparticles prepared with added Cu, Pd, and Ni. Environ. Sci. Technol. 2010, 44, 5079–5085. [Google Scholar] [CrossRef] [PubMed]
- Tee, Y.H.; Bachas, L.; Bhattacharyya, D. Degradation of trichloroethylene by iron-based bimetallic nanoparticles. J. Phys. Chem. C 2009, 113, 9454–9464. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.L.; Lien, H.L.; Koel, B.E.; Zhang, W.X. Iron nanoparticles for environmental clean-up: Recent development and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Quan, X.; Wang, J.X.; Zhang, Z.Y.; Chen, S. Rapid and complete dechlorination of PCP in aqueous solution using Ni-Fe nanoparticles under assistance of ultrasound. Chemosphere 2006, 65, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Lim, T.T. Catalytic reduction of chlorobenzenes with Pd/Fe nanoparticles: Reactive sites, catalyst stability, particle aging, and regeneration. Environ. Sci. Technol. 2007, 41, 7523–7529. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D. Degradation of toxic halogenated organic compounds by iron-containing mono-, bi- and tri-metallic particles in water. Inorg. Chimi. Acta 2015, 431, 48–60. [Google Scholar] [CrossRef]
- Choi, H.; Al-Abed, S.R.; Agarwal, S.; Dionysiou, D.D. Synthesis of reactive nano-Fe/Pd bimetallic system-impregnated activated carbon for the simultaneous adsorption and dechlorination of PCBs. Chem. Mater. 2008, 20, 3649–3655. [Google Scholar] [CrossRef]
- Sunkara, B.; Zhan, J.J.; Kolesnichenko, I.; Wang, Y.Q.; He, J.B.; Holland, J.E.; McPherson, G.L.; John, V.T. Modifying metal nanoparticle placement on carbon supports using an aerosol-based process, with application to the environmental remediation of chlorinated hydrocarbons. Langmuir 2011, 27, 7854–7859. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.J.; Kolesnichenko, I.; Sunkara, B.; He, J.B.; McPherson, G.L.; Piringer, G.; John, V.T. Multifunctional iron-carbon nanocomposites through an aerosol-based process for the in situ remediation of chlorinated hydrocarbons. Environ. Sci. Technol. 2011, 45, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Li, T.L.; Jin, Z.H. Stabilization of Fe0 nanoparticles with silica fume for enhanced transport and remediation of hexavalent chromium in water and soil. J. Environ. Sci. 2011, 23, 1211–1218. [Google Scholar] [CrossRef]
- Zheng, T.H.; Zhan, J.J.; He, J.B.; Day, C.; Lu, Y.F.; Mcpherson, G.L.; Piringer, G.; John, V.T. Reactivity characteristics of nanoscale zerovalent iron-silica composites for trichloroethylene remediation. Environ. Sci. Technol. 2008, 42, 4494–4499. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Yuranov, I.; Moeckli, P.; Suvorova, E.; Buffat, P.; Kiwi-Minsker, L.; Renken, A. Pd/SiO2 catalysts: Synthesis of Pd nanoparticles with the controlled size in mesoporous silicas. J. Mol. Catal. A Chem. 2003, 192, 239–251. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z.M. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Qiu, X.H.; Fang, Z.Q.; Liang, B.; Gu, F.L.; Xu, Z.C. Degradation of decabromodiphenyl ether by nano zero-valent iron immobilized in mesoporous silica microspheres. J. Hazard. Mater. 2011, 193, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.M.; Li, J.S.; Liu, C.; Shen, J.Y.; Sun, X.Y.; Han, W.Q.; Wang, L.J. Reduction of nitrobenzene using nanoscale zero-valent iron confined in channels of ordered mesoporous silica. Coll. Surf. A Physicochem. Eng. Asp. 2013, 425, 108–114. [Google Scholar] [CrossRef]
- Sun, X.; Yu, H.X.; Zheng, D.; Wang, X.S.; Li, J.S.; Wang, L.J. Incorporation of nanoscale zero-valent iron particles inside the channels of SBA-15 silica rods by a “two solvents” reduction technique. Appl. Surf. Sci. 2013, 279, 1–6. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Schmidt-Winkel, P.; Lukens, W.W., Jr.; Zhao, D.Y.; Yang, P.D.; Chmelka, B.F.; Stucky, G.D. Mesocellular siliceous foams with uniformly sized cells and windows. J. Am. Chem. Soc. 1999, 121, 254–255. [Google Scholar] [CrossRef]
- Taghavimoghaddam, J.; Knowles, G.P.; Chaffee, A.L. Preparation and characterization of mesoporous silica supported cobalt oxide as a catalyst for the oxidation of cyclohexanol. J. Mol. Catal. A Chem. 2012, 358, 79–88. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.M.; Yang, Q.H.; Yang, J.; Li, C. Morphological and structural evolution of mesoporous silicas in a mild buffer solution and lysozyme adsorption. Langmuir 2007, 23, 7255–7262. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.L.; Zhang, W.X. Nanoscale Pd/Fe bimetallic particles: Catalytic effects of palladium on hydrodechlorination. Appl. Catal. B Environ. 2007, 77, 110–116. [Google Scholar] [CrossRef]
- Schrick, B.; Blough, J.L.; Jones, A.D.; Mallouk, T.E. Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic nickel-iron nanoparticles. Chem. Mater. 2002, 14, 5140–5147. [Google Scholar] [CrossRef]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Qian, Y.; Wang, L.; Ge, Y.; Su, L.; Zhai, D.; Wang, J.; Wang, J.; Yu, J. Enhancement of Degradation and Dechlorination of Trichloroethylene via Supporting Palladium/Iron Bimetallic Nanoparticles onto Mesoporous Silica. Catalysts 2016, 6, 105. https://doi.org/10.3390/catal6070105
Wei J, Qian Y, Wang L, Ge Y, Su L, Zhai D, Wang J, Wang J, Yu J. Enhancement of Degradation and Dechlorination of Trichloroethylene via Supporting Palladium/Iron Bimetallic Nanoparticles onto Mesoporous Silica. Catalysts. 2016; 6(7):105. https://doi.org/10.3390/catal6070105
Chicago/Turabian StyleWei, Jianjun, Yajing Qian, Lutao Wang, Yijie Ge, Lingyan Su, Debin Zhai, Jiang Wang, Jing Wang, and Jiang Yu. 2016. "Enhancement of Degradation and Dechlorination of Trichloroethylene via Supporting Palladium/Iron Bimetallic Nanoparticles onto Mesoporous Silica" Catalysts 6, no. 7: 105. https://doi.org/10.3390/catal6070105
APA StyleWei, J., Qian, Y., Wang, L., Ge, Y., Su, L., Zhai, D., Wang, J., Wang, J., & Yu, J. (2016). Enhancement of Degradation and Dechlorination of Trichloroethylene via Supporting Palladium/Iron Bimetallic Nanoparticles onto Mesoporous Silica. Catalysts, 6(7), 105. https://doi.org/10.3390/catal6070105